- Open access
- Published: 28 September 2018

Posttraumatic stress disorder: from diagnosis to prevention
- Xue-Rong Miao ORCID: orcid.org/0000-0002-0665-8271 1 ,
- Qian-Bo Chen 1 ,
- Kai Wei 1 ,
- Kun-Ming Tao 1 &
- Zhi-Jie Lu 1
Military Medical Research volume 5 , Article number: 32 ( 2018 ) Cite this article
55k Accesses
44 Citations
18 Altmetric
Metrics details
Posttraumatic stress disorder (PTSD) is a chronic impairment disorder that occurs after exposure to traumatic events. This disorder can result in a disturbance to individual and family functioning, causing significant medical, financial, and social problems. This study is a selective review of literature aiming to provide a general outlook of the current understanding of PTSD. There are several diagnostic guidelines for PTSD, with the most recent editions of the DSM-5 and ICD-11 being best accepted. Generally, PTSD is diagnosed according to several clusters of symptoms occurring after exposure to extreme stressors. Its pathogenesis is multifactorial, including the activation of the hypothalamic–pituitary–adrenal (HPA) axis, immune response, or even genetic discrepancy. The morphological alternation of subcortical brain structures may also correlate with PTSD symptoms. Prevention and treatment methods for PTSD vary from psychological interventions to pharmacological medications. Overall, the findings of pertinent studies are difficult to generalize because of heterogeneous patient groups, different traumatic events, diagnostic criteria, and study designs. Future investigations are needed to determine which guideline or inspection method is the best for early diagnosis and which strategies might prevent the development of PTSD.
Posttraumatic stress disorder (PTSD) is a recognized clinical phenomenon that often occurs as a result of exposure to severe stressors, such as combat, natural disaster, or other events [ 1 ]. The diagnosis of PTSD was first introduced in the 3rd edition of the Diagnostic and Statistical Manual (DSM) (American Psychiatric Association) in 1980 [ 2 ].
PTSD is a potentially chronic impairing disorder that is characterized by re-experience and avoidance symptoms as well as negative alternations in cognition and arousal. This disease first raised public concerns during and after the military operations of the United States in Afghanistan and Iraq, and to date, a large number of research studies report progress in this field. However, both the underlying mechanism and specific treatment for the disease remain unclear. Considering the significant medical, social and financial problems, PTSD represents both to nations and to individuals, all persons caring for patients suffering from this disease or under traumatic exposure should know about the risks of PTSD.
The aim of this review article is to present the current understanding of PTSD related to military injury to foster interdisciplinary dialog. This article is a selective review of pertinent literature retrieved by a search in PubMed, using the following keywords: “PTSD[Mesh] AND military personnel”. The search yielded 3000 publications. The ones cited here are those that, in the authors’ view, make a substantial contribution to the interdisciplinary understanding of PTSD.
Definition and differential diagnosis
Posttraumatic stress disorder is a prevalent and typically debilitating psychiatric syndrome with a significant functional disturbance in various domains. Both the manifestation and etiology of it are complex, which has caused difficulty in defining and diagnosing the condition. The 3rd edition of the DSM introduced the diagnosis of PTSD with 17 symptoms divided into three clusters in 1980. After several decades of research, this diagnosis was refined and improved several times. In the most recent version of the DSM-5 [ 3 ], PTSD is classified into 20 symptoms within four clusters: intrusion, active avoidance, negative alterations in cognitions and mood as well as marked alterations in arousal and reactivity. The diagnosis requirement can be summarized as an exposure to a stressor that is accompanied by at least one intrusion symptom, one avoidance symptom, two negative alterations in cognitions and mood symptoms, and two arousal and reactivity turbulence symptoms, persisting for at least one month, with functional impairment. Interestingly, in the DSM-5, PTSD has been moved from the anxiety disorder group to a new category of ‘trauma- and stressor-related disorders’, which reflects the cognizance alternation of PTSD. In contrast to the DSM versions, the World Health Organization’s (WHO) International Classification of Diseases (ICD) has proposed a substantially different approach to diagnosing PTSD in the most recent ICD-11 version [ 4 ], which simplified the symptoms into six under three clusters, including constant re-experiencing of the traumatic event, avoidance of traumatic reminders and a sense of threat. The diagnosis requires at least one symptom from each cluster which persists for several weeks after exposure to extreme stressors. Both diagnostic guidelines emphasize the exposure to traumatic events and time of duration, which differentiate PTSD from some diseases with similar symptoms, including adjustment disorder, anxiety disorder, obsessive-compulsive disorder, and personality disorder. Patients with the major depressive disorder (MDD) may or may not have experienced traumatic events, but generally do not have the invasive symptoms or other typical symptoms that PTSD presents. In terms of traumatic brain injury (TBI), neurocognitive responses such as persistent disorientation and confusion are more specific symptoms. It is worth mentioning that some dissociative reactions in PTSD (e.g., flashback symptoms) should be recognized separately from the delusions, hallucinations, and other perceptual impairments that appear in psychotic disorders since they are based on actual experiences. The ICD-11 also recognizes a sibling disorder, complex PTSD (CPTSD), composed of symptoms including dysregulation, negative self-concept, and difficulties in relationships based on the diagnosis of PTSD. The core CPTSD symptom is PTSD with disturbances in self-organization (DSO).
In consideration of the practical applicability of the PTSD diagnosis, Brewin et al. conducted a study to investigate the requirement differences, prevalence, comorbidity, and validity of the DSM-5 and ICD-11 for PTSD criteria. According to their study, diagnostic standards for symptoms of re-experiencing are higher in the ICD-11 than the DSM, whereas the standards for avoidance are less strict in the ICD-11 than in the DSM-IV [ 5 ]. It seems that in adult subjects, the prevalence of PTSD using the ICD-11 is considerably lower compared to the DSM-5. Notably, evidence suggested that patients identified with the ICD-11 and DSM-5 were quite different with only partially overlapping cases; this means each diagnostic system appears to find cases that would not be diagnosed using the other. In consideration of comorbidity, research comparing these two criteria show diverse outcomes, as well as equal severity and quality of life. In terms of children, only very preliminary evidence exists suggesting no significant difference between the two. Notably, the diagnosis of young children (age ≤ 6 years) depends more on the situation in consideration of their physical and psychological development according to the DSM-5.
Despite numerous investigations and multiple revisions of the diagnostic criteria for PTSD, it remains unclear which type and what extent of stress are capable of inducing PTSD. Fear responses, especially those related to combat injury, are considered to be sufficient enough to trigger symptoms of PTSD. However, a number of other types of stressors were found to correlate with PTSD, including shame and guilt, which represent moral injury resulting from transgressions during a war in military personnel with deeply held moral and ethical beliefs. In addition, military spouses and children may be as vulnerable to moral injury as military service members [ 6 ]. A research study on Canadian Armed Forces personnel showed that exposure to moral injury during deployments is common among military personnel and represents an independent risk factor for past-year PTSD and MDD [ 7 ]. Unfortunately, it seems that pre- and post-deployment mental health education was insufficient to moderate the relationship between exposure to moral injury and adverse mental health outcomes.
In general, a large number of studies are focusing on the definition and diagnostic criteria of PTSD and provide considerable indicators for understanding and verifying the disease. However, some possible limitations or discrepancies continue to exist in current research studies. One is that although the diagnostic criteria for a thorough examination of the symptoms were explicit and accessible, the formal diagnosis of PTSD using structured clinical interviews was relatively rare. In contrast, self-rating scales, such as the Posttraumatic Diagnostic Scale (PDS) [ 8 ] and the Impact of Events Scale (IES) [ 9 ], were used frequently. It is also noteworthy that focusing on PTSD explicitly could be a limitation as well. The complexity of traumatic experiences and the responses to them urge comprehensive investigations covering all aspects of physical and psychological maladaptive changes.
Prevalence and importance
Posttraumatic stress disorder generally results in poor individual-level outcomes, including co-occurring disorders such as depression and substance use, and physical health problems. According to the DSM-5 reporting, more than 80% of PTSD patients share one or more comorbidities; for instance, the morbidity of PTSD with concurrent mild TBI is 48% [ 8 ]. Moreover, cognitive impairment has been identified frequently in PTSD. The reported incidence rate for PTSD ranges from 5.4 to 16.8% in military service members and veterans [ 10 , 11 , 12 , 13 , 14 ], which is almost double those in the general population. The estimated prevalence of PTSD varies depending on the group of patients studied, the traumatic events occurred, and the measurement method used (Table 1 ). However, it still reflects the profound effect of this mental disease, especially with the rise in global terrorism and military conflict in recent years. While PTSD can arise at any life stage in any population, most research in recent decades has focused on returned veterans; this means most knowledge regarding PTSD has come from the military population. Meanwhile, the impact of this disease on children has received scant attention.
The discrepancy of PTSD prevalence in males and females is controversial. In a large study of OEF/OIF veterans, the prevalence of PTSD in males and females was similar, although statistically more prevalent in men versus women (13% vs. 11%) [ 15 ]. Another study on the Navy and Marine Corps showed a slightly higher incidence for PTSD in the women compared to men (6.6% vs. 5.3%) [ 12 ]. However, the importance of combat exposure is unclear. Despite a lower level of combat exposure than male military personnel, females generally have considerably higher rates of military sexual trauma, which is significantly associated with the development of PTSD [ 16 ].
It is reported that 44–72% of veterans suffer high levels of stress after returning to civilian life. Many returned veterans with PTSD show emotion regulation problems, including emotion identification, expression troubles and self-control issues. Nevertheless, a meta-analytic investigation of 34 studies consistently found that the severity of PTSD symptoms was significantly associated with anger, especially in military samples [ 17 ]. Not surprisingly, high levels of PTSD and emotional regulation troubles frequently lead to poor family functioning or even domestic violence in veterans. According to some reports, parenting difficulties in veteran families were associated with three PTSD symptom clusters. Evans et al. [ 18 ] conducted a survey to evaluate the impact of PTSD symptom clusters on family functioning. According to their analysis, avoidance symptoms directly affected family functioning, whereas hyperarousal symptoms had an indirect association with family functioning. Re-experience symptoms were not found to impact family functioning. Notably, recent epidemiologic studies using data from the Veterans Health Administration (VHA) reported that veterans with PTSD were linked to suicide ideations and behaviors [ 19 ] (e.g., non-suicidal self-injury, NSSI), in which depression as well as other mood disruptions, often serve as mediating factors.
Previously, there was a controversial attitude toward the vulnerability of young children to PTSD. However, growing evidence suggests that severe and persistent trauma could result in stress responses worse than expected as well as other mental and physical sequelae in child development. The most prevalent traumatic exposures for young children above the age of 1 year were interpersonal trauma, mostly related to or derived from their caregivers, including witnessing intimate partner violence (IPV) and maltreatment [ 20 ]. Unfortunately, because of the crucial role that caregivers play in early child development, these types of traumatic events are especially harmful and have been associated with developmental maladaptation in early childhood. Maladaptation commonly represents a departure from normal development and has even been linked to more severe effects and psychopathology. In addition, the presence of psychopathology may interfere with the developmental competence of young children. Research studies have also broadened the investigation to sequelae of PTSD on family relationships. It is proposed that the children of parents with symptoms of PTSD are easily deregulated or distressed and appear to face more difficulties in their psychosocial development in later times compared to children of parents without. Meanwhile, PTSD veterans described both emotional (e.g., hurt, confusion, frustration, fear) and behavioral (e.g., withdrawal, mimicking parents’ behavior) disruption in their children [ 21 ]. Despite the increasing emphasis on the effects of PTSD on young children, only a limited number of studies examined the dominant factors that influence responses to early trauma exposures, and only a few prospective research studies have observed the internal relations between early PTSD and developmental competence. Moreover, whether exposure to both trauma types in early life is associated with more severe PTSD symptoms than exposure to one type remains an outstanding question.
Molecular mechanism and predictive factors
The mechanisms leading to posttraumatic stress disorder have not yet been fully elucidated. Recent literature suggests that both the neuroendocrine and immune systems are involved in the formulation and development of PTSD [ 22 , 23 ]. After traumatic exposures, the stress response pathways of the hypothalamic–pituitary–adrenal (HPA) axis and sympathetic nervous system are activated and lead to the abnormal release of glucocorticoids (GC) and catecholamines. GCs have downstream effects on immunosuppression, metabolism enhancement, and negative feedback inhibition of the HPA axis by binding to the GC receptor (GR), thus connecting the neuroendocrine modulation with immune disturbance and inflammatory response. A recent meta-analysis of 20 studies found increased plasma levels of proinflammatory cytokines tumor necrosis factor-alpha (TNF-a), interleukin-1beta (IL-1b), and interleukin-6 (IL-6) in individuals with PTSD compared to healthy controls [ 24 ]. In addition, some other studies speculate that there is a prospective association of C-reactive protein (CRP) and mitogen with the development of PTSD [ 25 ]. These findings suggest that neuroendocrine and inflammatory changes, rather than being a consequence of PTSD, may in fact act as a biological basis and preexisting vulnerability for developing PTSD after trauma. In addition, it is reported that elevated levels of terminally differentiated T cells and an altered Th1/Th2 balance may also predispose an individual to PTSD.
Evidence indicates that the development of PTSD is also affected by genetic factors. Research has found that genetic and epigenetic factors account for up to 70% of the individual differences in PTSD development, with PTSD heritability estimated at 30% [ 26 ]. While aiming to integrate genetic studies for PTSD and build a PTSD gene database, Zhang et al. [ 27 ] summarized the landscape and new perspective of PTSD genetic studies and increased the overall candidate genes for future investigations. Generally, the polymorphisms moderating HPA-axis reactivity and catecholamines have been extensively studied, such as FKBP5 and catechol-O-methyl-transferase (COMT). Other potential candidates for PTSD such as AKT, a critical mediator of growth factor-induced neuronal survival, were also explored. Genetic research has also made progress in other fields. For example, researchers have found that DNA methylation in multiple genes is highly correlated with PTSD development. Additional studies have found that stress exposure may even affect gene expression in offspring by epigenetic mechanisms, thus causing lasting risks. However, some existing problems in the current research of this field should be noted. In PTSD genetic studies, variations in population or gender difference, a wide range of traumatic events and diversity of diagnostic criteria all may attribute to inconsistency, thus leading to a low replication rate among similar studies. Furthermore, PTSD genes may overlap with other mental disorders such as depression, schizophrenia, and bipolar disorder. All of these factors indicate an urgent need for a large-scale genome-wide study of PTSD and its underlying epidemiologic mechanisms.
It is generally acknowledged that some mental diseases, such as major depressive disorder (MDD), bipolar disorder, and schizophrenia, are associated with massive subcortical volume change. Recently, numerous studies have examined the relationship between the morphology changes of subcortical structures and PTSD. One corrected analysis revealed that patients with PTSD show a pattern of lower white matter integrity in their brains [ 28 ]. Prior studies typically found that a reduced volume of the hippocampus, amygdala, rostral ventromedial prefrontal cortex (rvPFC), dorsal anterior cingulate cortex (dACC), and the caudate nucleus may have a relationship with PTSD patients. Logue et al. [ 29 ] conducted a large neuroimaging study of PTSD that compared eight subcortical structure volumes (nucleus accumbens, amygdala, caudate, hippocampus, pallidum, putamen, thalamus, and lateral ventricle) between PTSD patients and controls. They found that smaller hippocampi were particularly associated with PTSD, while smaller amygdalae did not show a significant correlation. Overall, rigorous and longitudinal research using new technologies, such as magnetoencephalography, functional MRI, and susceptibility-weighted imaging, are needed for further investigation and identification of morphological changes in the brain after a traumatic exposure.
Psychological and pharmacological strategies for prevention and treatment
Current approaches to PTSD prevention span a variety of psychological and pharmacological categories, which can be divided into three subgroups: primary prevention (before the traumatic event, including prevention of the event itself), secondary prevention (between the traumatic event and the development of PTSD), and tertiary prevention (after the first symptoms of PTSD become apparent). The secondary and tertiary prevention of PTSD has abundant methods, including different forms of debriefing, treatments for Acute Stress Disorder (ASD) or acute PTSD, and targeted intervention strategies. Meanwhile, the process of primary prevention is still in its infancy and faces several challenges.
Based on current research on the primary prevention of post-trauma pathology, psychological and pharmacological interventions for particular groups or individuals (e.g., military personnel, firefighters, etc.) with a high risk of traumatic event exposure were applicable and acceptable for PTSD sufferers. Of the studies that reported possible psychological prevention effects, training generally included a psychoeducational component and a skills-based component relating to stress responses, anxiety reducing and relaxation techniques, coping strategies and identifying thoughts, emotion and body tension, choosing how to act, attentional control, emotion control and regulation [ 30 , 31 , 32 ]. However, efficiency for these training has not been evaluated yet due to a lack of high-level evidence-based studies. Pharmacological options have targeted the influence of stress on memory formation, including drugs relating to the hypothalamic-pituitary-adrenal (HPA) axis, the autonomic nerve system (especially the sympathetic nerve system), and opiates. Evidence has suggested that pharmacological prevention is most effective when started before and early after the traumatic event, and it seems that sympatholytic drugs (alpha and beta-blockers) have the highest potential for primary prevention of PTSD [ 33 ]. However, one main difficulty limiting the exploration in this field is related to rigorous and complex ethical issues, as the application of pre-medication for special populations and the study of such options in hazardous circumstances possibly touches upon questions of life and death. Significantly, those drugs may have potential side effects.
There are several treatment guidelines for patients with PTSD produced by different organizations, including the American Psychiatric Association (APA), the United Kingdom’s National Institute for Health and Clinical Excellence (NICE), the International Society for Traumatic Stress Studies (ISTSS), the Institute of Medicine (IOM), the Australian National Health and Medical Research Council, and the Department of Veterans Affairs and Department of Defense (VA, DoD) [ 34 , 35 , 36 , 37 , 38 ]. Additionally, a large number of research studies are aiming to evaluate an effective treatment method for PTSD. According to these guidelines and research, treatment approaches can be classified as psychological interventions and pharmacological treatments (Fig. 1 ); most of the studies provide varying degrees of improvement in individual outcomes after standard interventions, including PTSD symptom reduction or remission, loss of diagnosis, release or reduction of comorbid medical or psychiatric conditions, quality of life, disability or functional impairment, return to work or to active duty, and adverse events.
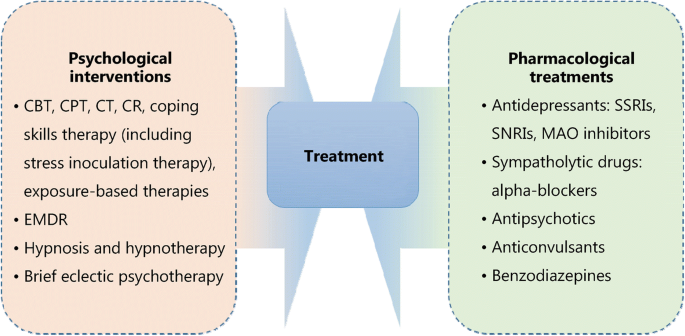
Psychological and pharmacological strategies for treatment of PTSD. CBT. Cognitive behavioral therapy; CPT. Cognitive processing therapy; CT. Cognitive therapy; CR. Cognitive restructuring; EMDR. Eye movement desensitization and reprocessing; SSRIs. Selective serotonin reuptake inhibitors; SNRIs. Serotonin and norepinephrine reuptake inhibitors; MAO. Monoamine oxidase
Most guidelines identify trauma-focused psychological interventions as first-line treatment options [ 39 ], including cognitive behavioral therapy (CBT), cognitive processing therapy (CPT), cognitive therapy (CT), cognitive restructuring (CR), coping skills therapy (including stress inoculation therapy), exposure-based therapies, eye movement desensitization and reprocessing (EMDR), hypnosis and hypnotherapy, and brief eclectic psychotherapy. These treatments are delivered predominantly to individuals, but some can also be conducted in family or group settings. However, the recommendation of current guidelines seems to be projected empirically as research on the comparison of outcomes of different treatments is limited. Jonas et al. [ 40 ] performed a systematic review and network meta-analysis of the evidence for treatment of PTSD. The study suggested that all psychological treatments showed efficacy for improving PTSD symptoms and achieving the loss of PTSD diagnosis in the acute phase, and exposure-based treatments exhibited the strongest evidence of efficacy with high strength of evidence (SOE). Furthermore, Kline et al. [ 41 ] conducted a meta-analysis evaluating the long-term effects of in-person psychotherapy for PTSD in 32 randomized controlled trials (RCTs) including 2935 patients with long-term follow-ups of at least 6 months. The data suggested that all studied treatments led to lasting improvements in individual outcomes, and exposure therapies demonstrated a significant therapeutic effect as well with larger effect sizes compared to other treatments.
Pharmacological treatments for PTSD include antidepressants such as selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), and monoamine oxidase (MAO) inhibitors, sympatholytic drugs such as alpha-blockers, antipsychotics, anticonvulsants, and benzodiazepines. Among these medications, fluoxetine, paroxetine, sertraline, topiramate, risperidone, and venlafaxine have been identified as efficacious in treatment. Moreover, in the Jonas network meta-analysis of 28 trials (4817 subjects), they found paroxetine and topiramate to be more effective for reducing PTSD symptoms than most other medications, whereas evidence was insufficient for some other medications as research was limited [ 40 ]. It is worth mentioning that in these studies, efficacy for the outcomes, unlike the studies of psychological treatments, was mostly reported as a remission in PTSD or depression symptoms; other outcomes, including loss of PTSD diagnosis, were rarely reported in studies.
As for the comparative evidence of psychological with pharmacological treatments or combinations of psychological treatments and pharmacological treatments with other treatments, evidence was insufficient to draw any firm conclusions [ 40 ]. Additionally, reports on adverse events such as mortality, suicidal behaviors, self-harmful behaviors, and withdrawal of treatment were relatively rare.
PTSD is a high-profile clinical phenomenon with a complicated psychological and physical basis. The development of PTSD is associated with various factors, such as traumatic events and their severity, gender, genetic and epigenetic factors. Pertinent studies have shown that PTSD is a chronic impairing disorder harmful to individuals both psychologically and physically. It brings individual suffering, family functioning disorders, and social hazards. The definition and diagnostic criteria for PTSD remain complex and ambiguous to some extent, which may be attributed to the complicated nature of PTSD and insufficient research on it. The underlying mechanisms of PTSD involve changes in different levels of psychological and molecular modulations. Thus, research targeting the basic mechanisms of PTSD using standard clinical guidelines and controlled interference factors is needed. In terms of treatment, psychological and pharmacological interventions could relief PTSD symptoms to different degrees. However, it is necessary to develop systemic treatment as well as symptom-specific therapeutic methods. Future research could focus on predictive factors and physiological indicators to determine effective prevention methods for PTSD, thereby reducing its prevalence and preventing more individuals and families from struggling with this disorder.
Abbreviations
American Psychiatric Association
Acute stress disorder
Cognitive behavioral therapy
Catechol-O-methyl-transferase
Cognitive processing therapy
Complex posttraumatic stress disorder
Cognitive restructuring
C-reactive protein
Cognitive therapy
Dorsal anterior cingulate cortex
Diagnostic and Statistical Manual
Disturbances in self-organization
Eye movement desensitization and reprocessing
Glucocorticoids
Glucocorticoids receptor
Hypothalamic–pituitary–adrenal axis
International classification of diseases
Impact of events scale
Interleukin-1beta
Interleukin-6
Institute of Medicine
Intimate partner violence
International Society for Traumatic Stress Studies
Monoamine oxidase
Major depressive disorder
United Kingdom’s National Institute for Health and Clinical Excellence
Non-suicidal self-injury
Posttraumatic diagnostic scale
Posttraumatic stress disorder
Randomized controlled trials
Rostral ventromedial prefrontal cortex
Serotonin and norepinephrine reuptake inhibitors;
Strength of evidence
Selective serotonin reuptake inhibitors
Tumor necrosis factor-alpha
DoD Department of Veterans Affairs and Department of Defense
Veterans Health Administration
World Health Organization
White J, Pearce J, Morrison S, Dunstan F, Bisson JI, Fone DL. Risk of post-traumatic stress disorder following traumatic events in a community sample. Epidemiol Psychiatr Sci. 2015;24(3):1–9.
Article Google Scholar
Kendell RE. Diagnostic and statistical manual of mental disorders, 3rd ed., revised (DSM-III-R). America J Psychiatry. 1980;145(10):1301–2.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-5. America J Psychiatry. 2013. doi: https://doi.org/10.1176/appi.books.9780890425596.744053 .
Maercker A, Brewin CR, Bryant RA, Cloitre M, Reed GM, Van OM, et al. Proposals for mental disorders specifically associated with stress in the international classification of Diseases-11. Lancet. 2013;381(9878):1683–5.
Brewin CR, Cloitre M, Hyland P, Shevlin M, Maercker A, Bryant RA, et al. A review of current evidence regarding the ICD-11 proposals for diagnosing PTSD and complex PTSD. Clin Psychol Rev. 2017;58(1): 1–15.
Google Scholar
Nash WP, Litz BT. Moral injury: a mechanism for war-related psychological trauma in military family members. Clin Child Fam Psychol Rev. 2013;16(4):365–75.
Nazarov A, Fikretoglu D, Liu A, Thompson M, Zamorski MA. Greater prevalence of post-traumatic stress disorder and depression in deployed Canadian Armed Forces personnel at risk for moral injury. Acta Psychiatr Scand. 2018;137(4):342–54.
Article CAS Google Scholar
Foa EB, Cashman L, Jaycox L, Perry K. The validation of a self-report measure of posttraumatic stress disorder: the posttraumatic diagnostic scale. Psychol Assess. 1997;9(9):445–51.
Gnanavel S, Robert RS. Diagnostic and statistical manual of mental disorders (5th edit) and the impact of events scale-revised. Chest. 2013;144(6):1974–5.
Reijnen A, Rademaker AR, Vermetten E, Geuze E. Prevalence of mental health symptoms in Dutch military personnel returning from deployment to Afghanistan: a 2-year longitudinal analysis. Eur Psychiatry. 2015;30(2):341–6.
Sundin J, Herrell RK, Hoge CW, Fear NT, Adler AB, Greenberg N, et al. Mental health outcomes in US and UK military personnel returning from Iraq. Br J Psychiatry. 2014;204(3):200–7.
Macera CA, Aralis HJ, Highfill-McRoy R, Rauh MJ. Posttraumatic stress disorder after combat zone deployment among navy and marine corps men and women. J Women's Health (Larchmt). 2014;23(6):499–505.
Macgregor AJ, Tang JJ, Dougherty AL, Galarneau MR. Deployment-related injury and posttraumatic stress disorder in US military personnel. Injury. 2013;44(11):1458–64.
Sandweiss DA, Slymen DJ, Leardmann CA, Smith B, White MR, Boyko EJ, et al. Preinjury psychiatric status, injury severity, and postdeployment posttraumatic stress disorder. Arch Gen Psychiatry. 2011;68(5):496–504.
Seal KH, Bertenthal D, Maguen S, Gima K, Chu A, Marmar CR. Getting beyond "Don't ask; don't tell": an evaluation of US veterans administration postdeployment mental health screening of veterans returning from Iraq and Afghanistan. Am J Public Health. 2008;98(4):714–20.
Street AE, Rosellini AJ, Ursano RJ, Heeringa SG, Hill ED, Monahan J, et al. Developing a risk model to target high-risk preventive interventions for sexual assault victimization among female U.S. army soldiers. Clin Psychol Sci. 2016;4(6):939–56.
Olatunji BO, Ciesielski BG, Tolin DF. Fear and loathing: a meta-analytic review of the specificity of anger in PTSD. Behav Ther. 2010;41(1):93–105.
Evans L, Cowlishaw S, Hopwood M. Family functioning predicts outcomes for veterans in treatment for chronic posttraumatic stress disorder. J Fam Psychol. 2009;23(4):531–9.
Mckinney JM, Hirsch JK, Britton PC. PTSD symptoms and suicide risk in veterans: serial indirect effects via depression and anger. J Affect Disord. 2017;214(1):100–7.
Briggsgowan MJ, Carter AS, Ford JD. Parsing the effects violence exposure in early childhood: modeling developmental pathways. J Pediatric Psychol. 2012;37(1):11–22.
Enlow MB, Blood E, Egeland B. Sociodemographic risk, developmental competence, and PTSD symptoms in young children exposed to interpersonal trauma in early life. J Trauma Stress. 2013;26(6):686–94.
Newport DJ, Nemeroff CB. Neurobiology of posttraumatic stress disorder. Curr Opin eurobiol. 2009;14(1 Suppl 1):13.
Neigh GN, Ali FF. Co-morbidity of PTSD and immune system dysfunction: opportunities for treatment. Curr Opin Pharmacol. 2016;29:104–10.
Passos IC, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J, et al. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry. 2015;2(11):1002.
Eraly SA, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A, et al. Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry. 2014;71(4):423.
Lebois LA, Wolff JD, Ressler KJ. Neuroimaging genetic approaches to posttraumatic stress disorder. Exp Neurol. 2016;284(Pt B):141–52.
Zhang K, Qu S, Chang S, Li G, Cao C, Fang K, et al. An overview of posttraumatic stress disorder genetic studies by analyzing and integrating genetic data into genetic database PTSD gene. Neurosci Biobehav Rev. 2017;83(1):647–56.
Bolzenius JD, Velez CS, Lewis JD, Bigler ED, Wade BSC, Cooper DB, et al. Diffusion imaging findings in US service members with mild traumatic brain injury and posttraumatic stress disorder. J Head Trauma Rehabil. 2018. https://doi.org/10.1097/HTR.0000000000000378 [Epub ahead of print].
Logue MW, Rooij SJHV, Dennis EL, Davis SL, Hayes JP, Stevens JS, et al. Smaller hippocampal volume in posttraumatic stress disorder: a multi-site ENIGMA-PGC study. Biol. Psychiatry . 2018;83(3):244–53.
Sijaric-Voloder S, Capin D. Application of cognitive behavior therapeutic techniques for prevention of psychological disorders in police officers. Health Med. 2008;2(4):288–92.
Deahl M, Srinivasan M, Jones N, Thomas J, Neblett C, Jolly A. Preventing psychological trauma in soldiers: the role of operational stress training and psychological debriefing. Brit J Med Psychol. 2000;73(1):77–85.
Wolmer L, Hamiel D, Laor N. Preventing children's posttraumatic stress after disaster with teacher-based intervention: a controlled study. J Am Acad Child Adolesc Psychiatry. 2011;50(4):340–8 348.e1–2.
Skeffington PM, Rees CS, Kane R. The primary prevention of PTSD: a systematic review. J Trauma Dissociation. 2013;14(4):404–22.
Jaques H. Introducing the national institute for health and clinical excellence. Eur Heart J. 2012;33(17):2111–2.
PubMed Google Scholar
Schnyder U. International Society for Traumatic Stress Studies (ISTSS). Psychosomatik Und Konsiliarpsychiatrie. 2008;2(4):261.
Bulger RE. The institute of medicine. Kennedy Inst Ethics J. 1992;2(1):73–7.
Anderle R, Brown DC, Cyran E. Department of Defense[C]. African Studies Association. 2011;2011:340–2.
Feussner JR, Maklan CW. Department of Veterans Affairs[J]. Med Care. 1998;36(3):254–6.
Sripada RK, Rauch SA, Liberzon I. Psychological mechanisms of PTSD and its treatment. Curr Psychiatry Rep. 2016;18(11):99.
Jonas DE, Cusack K, Forneris CA, Wilkins TM, Sonis J, Middleton JC, et al. Psychological and pharmacological treatments for adults with posttraumatic stress disorder (PTSD). Agency Healthcare Res Quality (AHRQ). 2013;4(1):1–760.
Kline AC, Cooper AA, Rytwinksi NK, Feeny NC. Long-term efficacy of psychotherapy for posttraumatic stress disorder: a meta-analysis of randomized controlled trials. Clin Psychol Rev. 2018;59:30–40.
Download references
Acknowledgments
We thank Jamie Bono for providing professional writing suggestions.
This work was supported by the National Natural Science Foundation of China (31371084 and 31171013 by ZJL), and the National Natural Science Foundation of China (81100276 by XRM).
Author information
Authors and affiliations.
Department of Anesthesiology and Intensive Care, Third Affiliated Hospital of Second Military Medical University, Shanghai, China
Xue-Rong Miao, Qian-Bo Chen, Kai Wei, Kun-Ming Tao & Zhi-Jie Lu
You can also search for this author in PubMed Google Scholar
Contributions
ZJL and XRM conceived the project. QBC, KW and KMT conducted the article search and acquisition. XRM and QBC analyzed the data. XRM wrote the manuscript. All the authors read and discussed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Xue-Rong Miao .
Ethics declarations
Ethics approval and consent to participate.
Not Applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Miao, XR., Chen, QB., Wei, K. et al. Posttraumatic stress disorder: from diagnosis to prevention. Military Med Res 5 , 32 (2018). https://doi.org/10.1186/s40779-018-0179-0
Download citation
Received : 20 March 2018
Accepted : 10 September 2018
Published : 28 September 2018
DOI : https://doi.org/10.1186/s40779-018-0179-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cognitive impairment
- Psychological interventions
- Neuroendocrine
Military Medical Research
ISSN: 2054-9369
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 14 September 2023
MDMA-assisted therapy for moderate to severe PTSD: a randomized, placebo-controlled phase 3 trial
- Jennifer M. Mitchell ORCID: orcid.org/0000-0002-7567-8129 1 , 2 , 3 ,
- Marcela Ot’alora G. ORCID: orcid.org/0000-0001-6174-7317 4 ,
- Bessel van der Kolk 5 ,
- Scott Shannon 6 ,
- Michael Bogenschutz ORCID: orcid.org/0000-0003-4530-3470 7 ,
- Yevgeniy Gelfand 8 ,
- Casey Paleos 9 ,
- Christopher R. Nicholas 10 ,
- Sylvestre Quevedo 2 , 11 ,
- Brooke Balliett 12 ,
- Scott Hamilton 13 ,
- Michael Mithoefer ORCID: orcid.org/0000-0002-4267-6135 14 ,
- Sarah Kleiman 15 ,
- Kelly Parker-Guilbert 16 ,
- Keren Tzarfaty 17 , 18 ,
- Charlotte Harrison 13 ,
- Alberdina de Boer 19 ,
- Rick Doblin 20 ,
- Berra Yazar-Klosinski 13 &
MAPP2 Study Collaborator Group
Nature Medicine volume 29 , pages 2473–2480 ( 2023 ) Cite this article
130k Accesses
35 Citations
2632 Altmetric
Metrics details
- Drug development
This multi-site, randomized, double-blind, confirmatory phase 3 study evaluated the efficacy and safety of 3,4-methylenedioxymethamphetamine-assisted therapy (MDMA-AT) versus placebo with identical therapy in participants with moderate to severe post-traumatic stress disorder (PTSD). Changes in Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) total severity score (primary endpoint) and Sheehan Disability Scale (SDS) functional impairment score (key secondary endpoint) were assessed by blinded independent assessors. Participants were randomized to MDMA-AT ( n = 53) or placebo with therapy ( n = 51). Overall, 26.9% (28/104) of participants had moderate PTSD, and 73.1% (76/104) of participants had severe PTSD. Participants were ethnoracially diverse: 28 of 104 (26.9%) identified as Hispanic/Latino, and 35 of 104 (33.7%) identified as other than White. Least squares (LS) mean change in CAPS-5 score (95% confidence interval (CI)) was −23.7 (−26.94, −20.44) for MDMA-AT versus −14.8 (−18.28, −11.28) for placebo with therapy ( P < 0.001, d = 0.7). LS mean change in SDS score (95% CI) was −3.3 (−4.03, −2.60) for MDMA-AT versus −2.1 (−2.89, −1.33) for placebo with therapy ( P = 0.03, d = 0.4). Seven participants had a severe treatment emergent adverse event (TEAE) (MDMA-AT, n = 5 (9.4%); placebo with therapy, n = 2 (3.9%)). There were no deaths or serious TEAEs. These data suggest that MDMA-AT reduced PTSD symptoms and functional impairment in a diverse population with moderate to severe PTSD and was generally well tolerated. ClinicalTrials.gov identifier: NCT04077437 .
Similar content being viewed by others

Microdosing with psilocybin mushrooms: a double-blind placebo-controlled study

Adults who microdose psychedelics report health related motivations and lower levels of anxiety and depression compared to non-microdosers

Major depressive disorder: hypothesis, mechanism, prevention and treatment
Post-traumatic stress disorder (PTSD) is a serious neuropsychiatric condition affecting approximately 5% of the US population each year 1 . Managing PTSD is particularly complicated in individuals experiencing the dissociative subtype of PTSD, recurrent exposure to trauma and comorbidities, such as mood disorders and alcohol and substance use disorders 2 , 3 , 4 . Together, these factors are associated with symptom exacerbation, treatment resistance and treatment discontinuation 3 , 5 . Trauma-focused psychotherapies are the gold standard treatment for PTSD. However, many individuals have persisting symptomology, and dropout rates are high 6 , 7 , 8 . Although the selective serotonin reuptake inhibitors (SSRIs) sertraline and paroxetine are FDA approved for treating PTSD, 35–47% of individuals do not respond to treatment 9 . More effective, therapeutic interventions are needed to address the immense individual, societal and economic burdens of PTSD 10 , 11 .
Mounting evidence supports substituted phenethylamine 3,4-methylenedioxymethamphetamine-assisted therapy (MDMA-AT) as a treatment for PTSD 12 , 13 . MDMA, an entactogen that promotes monoamine reuptake inhibition and release (primarily by inducing conformational change of pre-synaptic transporters 14 , 15 , 16 , 17 ), effectively modulates fear memory reconsolidation, enhances fear extinction and promotes openness and prosocial behavior 18 , 19 , 20 , 21 , 22 . Several phase 2 trials indicated that MDMA-AT has an acceptable risk–benefit profile in individuals with PTSD 13 . A pivotal phase 3 study (MAPP1) showed that MDMA-AT was generally well tolerated and met the trial’s primary and secondary endpoints of reduced PTSD symptom severity and decreased functional impairment 12 .
Due to disparities in trauma exposure, gender-diverse and transgender individuals, ethnoracial minorities, first responders, military personnel, veterans and victims of chronic sexual abuse have a disproportionately higher risk of developing PTSD 2 , 23 , 24 , 25 , 26 , 27 , 28 . However, these diverse populations are historically underrepresented in clinical trials 29 . Here we report the results of MAPP2, the second, confirmatory phase 3 study that extends the findings of MAPP1 (refs. 12 , 30 ) in an ethnoracially diverse population with moderate to severe PTSD (Supplementary Table 1 ).
Demographics and baseline characteristics
Participants were recruited from 21 August 2020 to 18 May 2022 (last participant visit on 2 November 2022). Overall, 324 individuals were screened, and 121 were enrolled. Of these, 17 individuals did not meet enrollment confirmation after initiation of preparation therapy, and 104 were confirmed for randomization: 53 were assigned to MDMA-AT and 51 to placebo with therapy (Fig. 1 ). Ninety-four participants completed the study, and nine discontinued ( n = 1 MDMA-AT; n = 8 placebo with therapy) (Fig. 1 and Supplementary Table 3 ).
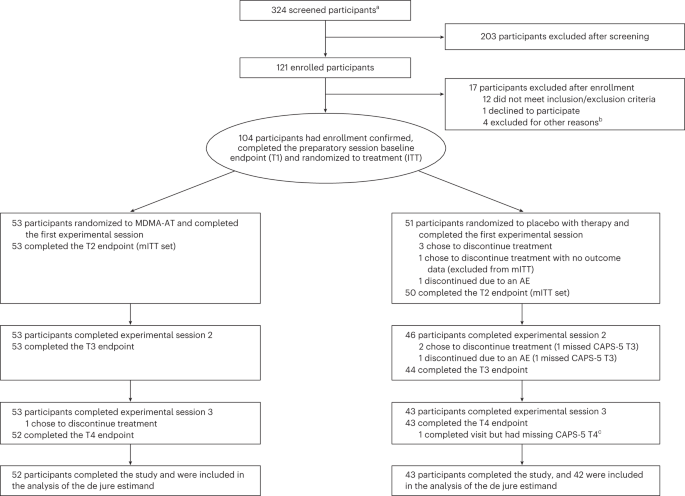
CONSORT diagram, indicating participant numbers and disposition throughout the course of the trial. Endpoint assessments (T1, T2, T3 and T4) of CAPS-5 and SDS were conducted after each experimental session. a The number of individuals after an initial phone screening who gave informed consent. b Other reasons for exclusion could include withdrawal of consent, adverse event or death, discontinuation of treatment by investigator, lack of therapeutic rapport and illness or lost to follow-up. c One participant in the placebo with therapy group completed the study but had missing item-level data on the final CAPS-5 assessment, and the final assessment was not included in the analysis of the de jure estimand. AE, adverse event; ITT, intention to treat; mITT, modified intention to treat; T, time of endpoint assessment; T1, baseline; T2, after experimental session 1; T3, after experimental session 2; T4, 6–8 weeks after experimental session 3 (18 weeks after baseline).
Baseline characteristics were generally similar between groups (Table 1 ). In total, 74 of 104 (71.2%) participants were assigned female sex at birth, with a higher proportion in the placebo with therapy group (42/51, 82.4%) than the MDMA-AT group (32/53, 60.4%). Participants were ethnically and racially diverse: 35 of 104 (33.7%) participants identified their race as other than White, and 28 of 104 (26.9%) identified their ethnicity as Hispanic/Latino. The mean (s.d.) duration of PTSD was 16.2 (13.3) years. The mean (s.d.) Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) score at baseline was 39.0 (6.6) and was similar between groups. Overall, 28 of 104 (26.9%) and 76 of 104 (73.1%) participants had moderate and severe PTSD, respectively; the dissociative subtype was present in 24 of 104 (23.1%) participants.
Primary outcomes
MDMA-AT significantly attenuated PTSD symptomology versus placebo with therapy, as measured by a reduction in CAPS-5 total severity score from baseline to 18 weeks. Mixed models for repeated measures (MMRM) analysis of the de jure estimand showed a least squares (LS) mean (95% confidence interval (CI)) change of −23.7 (−26.94, −20.44) for MDMA-AT versus −14.8 (−18.28, −11.28) for placebo with therapy (treatment difference: −8.9 (−13.70, −4.12), P < 0.001; Fig. 2a ). The Cohen’s d effect size of MDMA-AT versus placebo with therapy was d = 0.7; the within-group effect sizes were d = 1.95 for MDMA-AT and d = 1.25 for placebo with therapy. MMRM analysis of the de facto estimand revealed an LS mean change (95% CI) in CAPS-5 scores of −23.7 (−26.97, −20.47) for the MDMA-AT group versus −14.8 (−18.24, −11.33) for the placebo with therapy group ( P < 0.001).
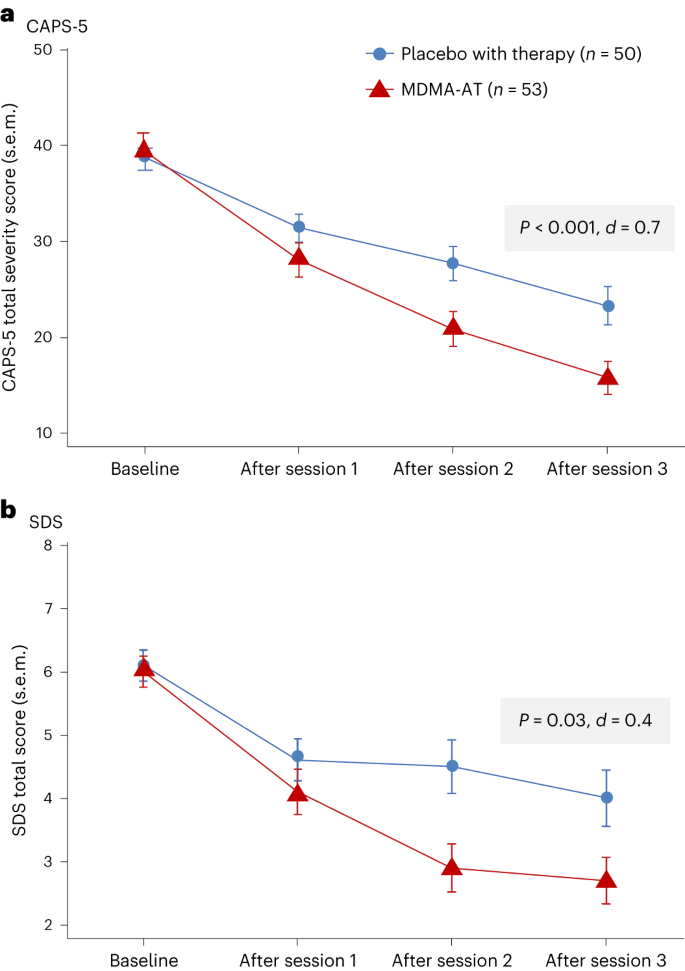
a , LS mean change (±s.e.m.) in CAPS-5 total severity score from baseline to after session 3 (primary outcome) for placebo with therapy ( n = 50) versus MDMA-AT ( n = 53, P < 0.001, Cohen’s d = 0.7). b , LS mean change (±s.e.m.) in SDS total score from baseline to after session 3 (key secondary outcome) for placebo with therapy ( n = 50) versus MDMA-AT ( n = 53, P = 0.03, Cohen’s d = 0.4).
Source data
Secondary outcomes.
MDMA-AT significantly mitigated clinician-rated functional impairment, as measured by a reduction in the Sheehan Disability Scale (SDS) from baseline. MMRM analysis of the de jure estimand revealed that the LS mean change (95% CI) in SDS total scores was −3.3 (−4.03, −2.60) with MDMA-AT versus −2.1 (−2.89, −1.33) with placebo with therapy (treatment difference: −1.20 (−2.26, −0.14); P = 0.03, d = 0.4; Fig. 2b ). Improvements were observed across all domains, including family life, social life and work life (Supplementary Table 4 ).
Exploratory outcomes
In the MDMA-AT group, 45 of 52 (86.5%) participants were responders with a clinically meaningful improvement at 18 weeks after baseline, defined as a ≥10-point reduction in CAPS-5 total severity score, versus 29 of 42 (69.0%) in the placebo with therapy group (Fig. 3 ). By study end, 37 of 52 (71.2%) participants in the MDMA-AT group no longer met DSM-5 criteria for PTSD versus 20 of 42 (47.6%) participants in the placebo with therapy group. Furthermore, 24 of 52 (46.2%) participants in the MDMA-AT group and nine of 42 (21.4%) participants in the placebo with therapy group met remission criteria (Fig. 3 ). The net number of participants needed to treat for each responder analysis group was as follows: responder, six; non-responder, six; loss of diagnosis, four; remission, four.
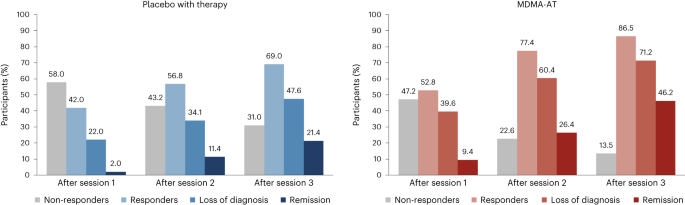
A ≥10-point reduction in CAPS-5 total severity score was considered to be clinically meaningful. Responders (≥10-point reduction from baseline), loss of diagnosis (≥10-point reduction from baseline and no longer meeting PTSD diagnostic criteria) and remission (loss of diagnosis and CAPS-5 total severity score of 11 or less) were tracked in both groups as a percentage of participants. Non-responders were defined as any CAPS-5 total severity score change <10-point reduction from baseline.
Covariate analyses demonstrated similar responses to treatment regardless of disease severity, risk of hazardous alcohol or substance use disorder, severe adverse childhood experiences or dissociative subtype PTSD. The only measured exploratory covariate with a significant interaction with treatment was lifetime history of SSRI use, which was associated with improved efficacy of MDMA-AT ( P = 0.02; Supplementary Table 5 ). Covariates significantly impacting the main effect were sex assigned at birth and baseline Beck Depression Inventory (BDI)-II score; female sex assigned at birth and baseline BDI-II score ≥23 were both associated with improved outcomes irrespective of treatment assignment ( P < 0.05).
A blinding survey conducted at study termination showed that 33 of 44 (75.0%) participants in the placebo with therapy group were certain or thought they received placebo, whereas nine of 44 (20.5%) participants inaccurately thought that they received MDMA, and two of 44 (4.5%) participants could not tell. In the MDMA-AT group, 49 of 52 (94.2%) participants were certain or thought that they received MDMA; one of 52 (1.9%) participants inaccurately thought that they received placebo; and two of 52 (3.8%) participants could not tell (Supplementary Table 6 ). When asked for the reason for their belief in treatment assignment, most participants in the MDMA-AT group reported attributing their response on the blinding survey to experiencing positive mental or emotional effect (45/52 (86.5%)) and positive physical effect (29/52 (55.8%)), whereas most of the participants in the placebo with therapy group reported experiencing no effect (28/44 (63.6%)).
Most participants (102/104, 98.1%) experienced at least one treatment-emergent adverse event (TEAE) during the study (Table 2 ); seven experienced a severe TEAE (MDMA-AT, n = 5 (9.4%); placebo with therapy, n = 2 (3.9%)). None had a serious TEAE. Two participants (3.9%) in the placebo with therapy group discontinued treatment due to TEAEs. Frequently reported TEAEs (occurring with incidence >10% and at least twice the prevalence in the MDMA-AT group versus the placebo with therapy group) included muscle tightness, nausea, decreased appetite and hyperhidrosis (Table 2 ). These were mostly transient and of mild or moderate severity. At least one treatment-emergent adverse event of special interest (TEAESI) occurred in six of 53 (11.3%) participants in the MDMA-AT group and three of 51 (5.9%) participants in the placebo with therapy group (Table 2 ). No TEAESIs of MDMA abuse, misuse, physical dependence or diversion were reported.
Eight participants (MDMA-AT, n = 7; placebo with therapy, n = 1) experienced cardiac TEAEs, which included palpitations (MDMA-AT, n = 5 (9.4%); placebo with therapy, n = 1 (2.0%)) and tachycardia (MDMA-AT, n = 2 (3.8%)); all were mild. Nine participants (MDMA-AT, n = 7; placebo with therapy, n = 2) experienced vascular TEAEs; all were mild, except for one participant in the MDMA-AT group who had a history of hypertension, who was not taking anti-hypertensive medications and who experienced a TEAE of moderate hypertension (Supplementary Table 7 ). Five participants had cardiac TEAESIs: four participants in the MDMA-AT group and one participant in the placebo with therapy group reported palpitations (Supplementary Table 7 ). Participants in the MDMA-AT group experienced temporary dose-dependent increases in mean blood pressure (BP) and pulse during experimental sessions compared to the placebo with therapy group (Supplementary Table 8 ).
Transient increases in heart rate and BP were expected and were observed during experimental sessions in a dose-dependent manner. Greater fluctuations in BP were seen during experimental sessions 2 and 3 in the participants treated with MDMA, most likely due to the higher doses of MDMA administered. These transient elevations did not require clinical intervention, including among the subset of participants with well-controlled hypertension. Because the current dosing regimen involves administering a single, split drug dose under observation, for a limited number of times, each after a lengthy washout, cardiovascular risk is likely to have been sufficiently mitigated by the study procedures and screening measures.
Psychiatric TEAEs occurred at a similarly high frequency in both groups (MDMA-AT, n = 44 (83.0%); placebo with therapy, n = 37 (72.5%)), with suicidal ideation, insomnia and anxiety reported most frequently. Psychiatric TEAEs were mostly mild to moderate; three severe events occurred in the MDMA-AT group (5.7%; n = 1 each: dissociation, flashback and grief reaction) and two in the placebo with therapy group (3.9%; n = 1 each: agitation and anxiety). No severe TEAEs of suicidal ideation or behavior were reported. Two participants in the MDMA-AT group had suicidality TEAESIs of suicidal ideation, one of whom engaged in non-suicidal self-injurious behavior. Two participants in the placebo with therapy group had suicidality TEAESIs; one engaged in non-suicidal self-injurious behavior, and one had suicidal ideation and trichotillomania (Supplementary Table 9 ).
More than 80% (87/104) of participants had a lifetime history of suicidal ideation; 13 of 53 (24.5%) in the MDMA-AT group and 12 of 51 (23.5%) in the placebo with therapy group reported suicidal ideation during the final preparation session (V4). The number of participants reporting positive suicidal ideation varied throughout the study but collectively never exceeded baseline values in either group (Supplementary Fig. 2 ). Three participants (two MDMA-AT and one placebo with therapy) had treatment-emergent active suicidal ideation with at least some intent to act (Columbia-Suicide Severity Rating Scale (C-SSRS) score of 4 or 5), which was observed on five occasions (MDMA-AT, three events; placebo with therapy, two events) (Supplementary Fig. 2 ). Of these, one participant in the MDMA-AT group with no suicidal ideation at baseline had the emergence of active suicidal ideation with at least some intent to act.
In this confirmatory phase 3 study of participants with moderate to severe PTSD, MDMA-AT significantly improved PTSD symptoms and functional impairment, as assessed by CAPS-5 and SDS, respectively, compared to placebo with therapy over 18 weeks. Notably, 45 of 52 (86.5%) participants treated with MDMA-AT achieved a clinically meaningful benefit, and 37 of 52 (71.2%) participants no longer met criteria for PTSD by study end. In a historic first, to our knowledge, for psychedelic treatment studies, participants who identified as ethnically or racially diverse encompassed approximately half of the study sample. These findings confirm and extend the results observed in MAPP1 (ref. 12 ), with general consistency across endpoints.
Given the diverse population and degree of participant complexity, the replication of efficacy is particularly notable. In our study, 26.9% (28/104) of participants expressed moderate PTSD, whereas, in MAPP1, all participants expressed severe PTSD 12 . A substantial proportion of participants displayed comorbid features associated with high treatment resistance 5 , such as major depression, multiple sources of trauma (including childhood and combat trauma) and dissociative subtype PTSD. In keeping with MAPP1, treatment was not significantly affected by disease severity, risk of hazardous alcohol or substance use disorder, severe adverse childhood experiences or dissociative subtype. Furthermore, there was no observed site-to-site variability and no differential effect if participants stayed overnight after the experimental session. However, lifetime history of SSRIs, female sex assigned at birth and BDI-II score ≥23 at baseline were associated with positive impacts on outcomes and may warrant further study based on the exploratory nature of these analyses.
MDMA simultaneously induces prosocial feelings and softens responses to emotionally challenging and fearful stimuli 19 , potentially enhancing the ability of individuals with PTSD to benefit from psychotherapy by reducing sensations of fear, threat and negative emotionality 18 , 19 . The low dropout rate for MDMA-AT has been replicated across seven studies, suggesting that MDMA induces a true shift in participant engagement 12 , 13 . In contrast, a recent study comparing psychotherapies in veterans with PTSD reported dropout rates of 55.8% and 46.6% for prolonged exposure and cognitive processing therapy, respectively 31 . The MAPP2 dropout rate was 1.9% (1/53) in the MDMA-AT group and 15.7% (8/51) in the placebo with therapy group. The higher proportion of dropouts in the placebo with therapy group relative to MDMA-AT could be attributed to participants receiving less effective treatment and to disappointment from ineffective therapeutic blinding, although blinding survey data showed that not all participants correctly identified the treatment that they received.
Consistent with MAPP1, no new major safety issues were reported. Common TEAEs were similar to previous studies and consistent with expected effects of MDMA 12 , 32 . Rates of cardiac TEAEs were low, and increases in BP and pulse were mild, transient and consistent with MDMA’s sympathomimetic effects 18 , 33 , 34 . Consistent with PTSD, suicidal ideation was observed in both groups. MDMA did not appear to increase this risk, and no suicidal behavior was observed. C-SSRS scores varied throughout the study but never exceeded baseline values for either group. Notably, there were five total events of treatment-emergent C-SSRS scores of 4 or 5: three in the MDMA-AT group and two in the placebo with therapy group. MAPP2 enrolled participants with a history of suicidality but excluded those with a current, serious imminent suicide risk; thus, special attention to this vulnerable population is warranted in future studies. In alignment with MAPP1 (ref. 12 ), there were no reports of problematic MDMA abuse or dependence, including in participants with histories of, or current, alcohol and substance use disorders. However, it is important to note that participants with any substance use disorder other than cannabis or alcohol in the 12 months before enrollment were excluded from MAPP2, as were participants with severe or moderate (in early remission) alcohol or cannabis use disorder. However, exploratory findings from the MAPP1 phase 3 trial indicated that MDMA-AT was actually associated with a significantly greater reduction in mean Alcohol Use Disorder Identification Test change scores compared to placebo with therapy, suggesting that the effects of MDMA-AT on hazardous alcohol use secondary to PTSD should be further studied 35 . Long-term data are also needed to assess the risk of MDMA abuse or misuse after study participation.
Although the sample sizes of the MAPP1 and MAPP2 phase 3 studies had 90% statistical power and were developed with guidance from the FDA to ensure adequate, rigorous testing of outcomes, these evaluations did not extend further than 2 months after therapy and were intended to support an acute treatment course. To support these studies, data from the ongoing follow-up of participants from phase 2 and 3 studies (ClinicalTrials.gov Identifier: NCT05066282 ) will be important for further assessment of the long-term effectiveness of MDMA-AT in participants with PTSD. It is of interest to note that pooled phase 2 analyses of participants with at least 12 months of follow-up after their final MDMA-AT session have shown that LS mean CAPS-IV scores continue to improve between the final session and follow-up 32 .
Several limitations may impact the integration of MDMA-AT into clinical care, including the exclusion of participants with high suicide risk, comorbid personality disorders and underlying cardiovascular disease. Observed effect sizes for MDMA-AT (between-group, d = 0.7; within-subject, d = 1.95) were similar to MAPP1 (ref. 12 ) (between-group, d = 0.91; within-subject, d = 2.1), and, although higher than those observed in SSRI studies (ranging from 0.09 to 0.56 versus placebo for sertraline and paroxetine 36 ), the superiority of MDMA-AT over SSRIs cannot be assumed without a direct comparison. The complex relationship between SSRI use/history and MDMA-AT treatment efficacy was beyond the scope of the current statistical analysis plan and sample size but will be important to consider in future studies. In addition, further study of MDMA with other forms of psychotherapy for PTSD should be explored.
The notable effect seen in the placebo with therapy arm could suggest the standalone value of the manualized inner-directed therapy that was developed for use with MDMA. Additional head-to-head studies will need to be conducted to evaluate whether this form of manualized therapy provides greater value in the treatment of PTSD than the current first-line cognitive behavioral therapy and prolonged exposure therapy treatments 37 .
Although treatment expectancy, per se, was not measured in this study, prospective treatment expectancy would likely have been high in both study arms, with random assignment expected to distribute this equally between groups. Although expectancy effects are a well-known issue in psychiatric clinical trials and are intertwined with the observation of treatment benefit during a trial 38 , several observations support expectancy mitigation in the current study: (1) the groups did not separate after the first experimental session; (2) placebo with therapy dropouts did not uniformly occur after the first experimental session; and (3) blinding survey data (Supplementary Table 6 ) showed that not all participants correctly identified the treatment that they received.
The therapists who participated in this study were required to complete the sponsor’s training program (see Supplementary Methods for further details). To ensure consistent clinical practice and to mitigate harm, it may be of benefit for prescribers to complete additional training and continuing education if MDMA-AT is approved for use by a regulatory agency.
This confirmatory phase 3 trial showed consistent benefits of MDMA-AT in an ethnoracially diverse group of individuals with longstanding moderate to severe PTSD and numerous comorbidities. The dropout rate was low, and treatment was generally well tolerated. These findings represent the culmination of over two decades of research 39 , and, together with MAPP1, indicate that further consideration of this treatment in individuals with moderate to severe PTSD is warranted.
Study design and oversight
This multi-site, randomized, double-blind, placebo-controlled study assessed the efficacy and safety of MDMA-AT versus placebo with therapy in participants diagnosed with moderate or severe PTSD ( NCT04077437 ). Thirteen study sites (11 in the United States and two in Israel, both institutional and private) participated. The trial was conducted in accordance with the Good Clinical Practice guidelines of the International Council for Harmonization and with the ethical principles of the Declaration of Helsinki. An independent data monitoring committee ensured that the study was conducted safely and had sufficient sample size. The review boards and institutions that approved the study protocol are listed in the Supplementary Methods .
Participants
After written informed consent, participants were screened for eligibility. Adults (≥18 years of age) meeting the full DSM-5 criteria for current PTSD per CAPS-5 assessment 40 , 41 and a CAPS-5 total severity score ≥28 (moderate or higher severity) with symptom duration of ≥6 months were eligible for enrollment confirmation. During the Preparation Period that preceded the Treatment Period, participants were tapered off all psychiatric medications before baseline to avoid potential drug interactions and confounding efficacy (Supplementary Fig. 1 ). Full inclusion and exclusion criteria are outlined in the Supplementary Methods .
Randomization and masking
Participants were randomized in a 1:1 allocation and in a blinded fashion to the MDMA-AT and placebo with therapy groups, stratified by clinical site. Randomization was managed via an interactive web randomization system (IWRS) (IT Clinical version 11.0.1) based on a centralized randomization schedule developed by an independent third-party vendor to maintain blinding.
A central pool of blinded independent assessors was used to mitigate the risk of functional unblinding 42 . Assessors were trained and supervised by independent consultants with expertise in PTSD diagnostics and the CAPS-5 to ensure inter-rater reliability and validity of assessments. Supervision involved reviewing each assessor’s first two assessments as well as 20% of all assessments (chosen at random) throughout the study, with each review resulting in detailed feedback for the assessor. The independent assessors were blinded to the general study design, study visit, treatment assignment, number of treatments received and any safety data for the participant. Participants were instructed to withhold their opinion on treatment group assignment and the number of completed visits from the independent assessors. Each assessor conducted no more than one CAPS-5 assessment with each participant to reduce potential bias and expectancy effect from having conducted repeat CAPS-5s with a participant. Assessors were also vetted before their onboarding to ensure that there were no conflicts of interest (such as other involvement within the Multidisciplinary Association for Psychedelic Studies (MAPS) organization or a bias toward MDMA-AT), and assessors were instructed to not expose themselves to scholarly presentations and papers related to MDMA-AT for PTSD to maintain their blinding to study design.
To ensure that all site and sponsor staff were shielded from study outcomes, the blinded independent assessor pool collected and stored outcome measures in a dedicated database that was separate from the blinded clinical database. A blinding survey was conducted at study termination (visit 20) to assess if participants thought that they received MDMA or placebo.
Trial procedures were consistent with MAPP1 (ref. 12 ). Enrolled participants underwent three 90-min preparation sessions with a two-person therapy team, including at least one licensed therapist, and were then randomized 1:1 to receive MDMA-AT or placebo with therapy for approximately 3 months. The treatment period consisted of three 8-h dosing sessions, in conjunction with therapy, spaced approximately 1 month apart. Therapy was conducted by trained personnel in accordance with the MAPS MDMA-AT treatment manual ( https://maps.org/treatment-manual ) and trial protocol. During experimental sessions, and in keeping with the dosing in MAPP1, participants received a split dose of 120–180 mg of MDMA or placebo. For the first experimental session, the initial dose of 80 mg was followed by a supplemental half-dose of 40 mg. In the second and third experimental sessions, the initial dose of 120 mg was followed by a supplemental half-dose of 60 mg. The supplemental half dose was administered 1.5–2 h after the initial dose. Participants in both treatment groups received identical therapy. The 120-mg (80 mg + 40 mg) split dose was selected for the first experimental session in phase 3 trials to allow patients to acclimate to the treatment regimen using a clinical titration approach based on clinician recommendations from a phase 2 trial in veterans and first responders 13 . During the second and third experimental sessions, doses were escalated to 180 mg (120 mg + 60 mg), as this was the most frequently studied efficacious dose in phase 2 trials. This dosing regimen also provides clinicians with the option of dose adjustments if needed.
Within the MDMA-AT group, three participants did not undergo dose escalation in experimental sessions 2 and 3, and two participants experienced dose administration timing errors (Supplementary Table 2 ). Each experimental session was followed by three 90-min integration sessions to support participants in processing and understanding their experience (Supplementary Fig. 1 ). Full procedures, including details on therapy teams and training, are outlined in the Supplementary Methods .
Independent assessors conducted CAPS-5 and SDS outcome assessments at baseline, after experimental sessions 1 and 2 and 6–8 weeks after experimental session 3 (18 weeks after baseline) via video interviews. Primary and secondary objectives were mean change in CAPS-5 total severity and SDS scores, respectively, for MDMA-AT versus placebo with therapy from baseline to 18 weeks after baseline.
Exploratory outcome measurements included characterization of the treatment response and differences between the treatment groups by demographics and characteristics. Responder analyses were based on categorical diagnostic assessment data and the CAPS-5 total severity score assessment. PTSD severity was defined using the CAPS-5 total severity score as follows: asymptomatic (0–10), mild (11–22), moderate (23–34), severe (35–46) and extreme (47+) (ref. 41 ). A ≥10-point reduction in CAPS-5 total severity score was considered to be clinically meaningful as agreed upon with the FDA through a Special Protocol Assessment. Four responder categories were derived and compared at each post-experimental session visit using CAPS-5 scores. These categories were: non-responder (<10-point reduction from baseline), responder (≥10-point reduction from baseline), loss of diagnosis (≥10-point reduction from baseline and no longer meeting PTSD diagnostic criteria) and remission (CAPS-5 total severity score of 11 or less and no longer meeting PTSD diagnostic criteria).
Safety objectives included assessment of differences between groups in severity, incidence and frequency of TEAEs, serious TEAEs, TEAESIs, suicidal ideation and behavior and vital signs. TEAEs were defined as any adverse event that occurred during the treatment period from the first experimental session to the last integration session. The severity of TEAEs was determined by the site physician as mild (no limitation in normal daily activity), moderate (some limitation in normal daily activity) or severe (unable to perform normal daily activity). A serious TEAE was defined as any unforeseen medical event at any dose of the drug that resulted in death; was life-threatening; required inpatient hospitalization; caused significant disability or incapacity; resulted in a congenital anomaly or birth defect; or required intervention to prevent permanent impairment or damage. Serious TEAEs also included any event, based on medical judgement, that jeopardized the participant or may have required intervention to prevent one of the events listed previously. With the exception of serious adverse event reporting, relatedness to study drug was not assessed by investigators, to preserve blinding. In an effort to identify common adverse events that may be most related to MDMA, TEAEs occurring with incidence >10% and at least twice the prevalence in the MDMA-AT group versus the placebo with therapy group are reported. Suicidality was tracked at each study visit using the C-SSRS (see the Supplementary Methods for more information).
Statistical analysis
SAS version 9.4 (SAS Institute) was used for analyses. Sample size was calculated to achieve a power of 90% at an alpha of 0.0499.
Efficacy was tested using an MMRM analysis comparing the change from baseline to 18 weeks after baseline in CAPS-5 and SDS scores between treatment groups in two-sided tests with alpha set at 0.0499. The alpha was adjusted to account for an administrative interim analysis for sample size re-estimation conducted after all participants were enrolled and 60% of primary endpoint data had been collected. Fixed effects were treatment, visit, treatment group by visit interaction and dissociative subtype; baseline CAPS-5 score was a covariate. Primary and secondary efficacy analyses used a de jure (related to initially randomized treatment) estimand and a supportive de facto (treatment policy) estimand of the modified intention-to-treat population, which required exposure to MDMA or placebo and at least one follow-up CAPS-5 assessment, as in MAPP1 (ref. 12 ). The de jure dataset included all available data, except for 12 (one MDMA-AT and 11 placebo with therapy) outcome measurements taken after treatment discontinuation in analysis of treatment efficacy (Supplementary Table 3 ). Missed observations were considered missing at random (MAR), and choice of this assumption was tested with a tipping point analysis ( Supplementary Methods ).
In additional exploratory analyses, 13 covariates were assessed in the model, with alpha set at 0.0499: age, sex (self-reported), prior use of selective SSRIs, work disability, disease severity, PTSD duration, dissociative subtype, overnight site stay, site ID, moderate depression (as measured by the BDI-II), severe adverse childhood experiences and moderate alcohol and substance use disorder risk (as measured by the Drug Use Disorders Identification Test and the Alcohol Use Disorders Identification Test). Analyses of primary or secondary outcomes by gender were not planned a priori; some exploratory analyses included sex as a covariate ( Supplementary Methods ).
Safety analysis evaluated TEAEs at the participant level, including all participants who received MDMA or placebo. Causal association with MDMA was determined based on relative incidence of TEAEs with at least a two-fold difference between groups.
Adverse events of special interest
In accordance with FDA guidance, special attention was paid to a subset of adverse events, TEAESIs, relating to cardiac function, suicide risk and MDMA abuse, misuse or diversion. TEAESIs involving cardiac function that could be indicative of QT prolongation or cardiac arrhythmias were collected, including torsade de pointes, sudden death, ventricular extrasystoles, ventricular tachycardia, ventricular fibrillation and flutter, non-postural syncope and seizures. TEAESIs involving suicide risk included suicide, suicide attempts, self-harm associated with suicidal ideation, suicide ideation assessed as a score of 4 or 5 on the C-SSRS and suicidal ideation judged by the investigator to be serious/severe. TEAESIs involving terms of MDMA abuse, misuse, drug diversion, dependence or overdose were also collected.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available from the sponsor beginning 1 year after completion of the trial. However, restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available. Data are, however, available from the authors upon reasonable request and with the permission of the sponsor. All requests for raw and analyzed data are promptly reviewed to verify if the request is subject to any confidentiality obligations. Participant-related data not included in the paper were generated as part of clinical trials and may be subject to participant confidentiality. Any data that can be shared will be released via a data use agreement. Proposals should be directed to https:/maps.org/datause . Source data are provided with this paper.
Code availability
Commercially available software (SAS version 9.4 or higher, SAS Institute) was used for analyses, in keeping with the statistical analysis plan.
US Department of Veteran Affairs. PTSD: National Center for PTSD. How common is PTSD in adults? ( https://www.ptsd.va.gov/understand/common/common_adults.asp ).
de Silva, U., Glover, N. & Katona, C. Prevalence of complex post-traumatic stress disorder in refugees and asylum seekers: systematic review. BJPysch Open 7 , e194 (2021).
Article Google Scholar
de Castro Longo, M. S. et al. Comorbidity in post-traumatic stress disorder: a population-based study from the two largest cities in Brazil. J. Affect. Disord. 263 , 715–721 (2020).
Hill, S. B. et al. Dissociative subtype of posttraumatic stress disorder in women in partial and residential levels of psychiatric care. J. Trauma Dissociation 21 , 305–318 (2020).
Article PubMed Google Scholar
Roberts, N. P., Lotzin, A. & Schäfer, I. A systematic review and meta-analysis of psychological interventions for comorbid post-traumatic stress disorder and substance use disorder. Eur. J. Psychotraumatol. 13 , 2041831 (2022).
Article PubMed PubMed Central Google Scholar
Lewis, C., Roberts, N. P., Gibson, S. & Bisson, J. I. Dropout from psychological therapies for post-traumatic stress disorder (PTSD) in adults: systematic review and meta-analysis. Eur. J. Psychotraumatol. 11 , 1709709 (2020).
Steenkamp, M. M., Litz, B. T., Hoge, C. W. & Marmar, C. R. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA 314 , 489–500 (2015).
Article CAS PubMed Google Scholar
Mavranezouli, I. et al. Psychological treatments for post-traumatic stress disorder in adults: a network meta-analysis. Psychol. Med. 50 , 542–555 (2020).
Alexander, W. Pharmacotherapy for post-traumatic stress disorder in combat veterans: focus on antidepressants and atypical antipsychotic agents. Pharm. Ther. 37 , 32 (2012).
Google Scholar
Davis, L. L. et al. The economic burden of posttraumatic stress disorder in the United States from a societal perspective. J. Clin. Psychiatry 83 , 21m14116 (2022).
Couette, M., Mouchabac, S., Bourla, A., Nuss, P. & Ferreri, F. Social cognition in post-traumatic stress disorder: a systematic review. Br. J. Clin. Psychol. 59 , 117–138 (2020).
Mitchell, J. M. et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat. Med. 27 , 1025–1033 (2021).
Article CAS PubMed PubMed Central Google Scholar
Mithoefer, M. C. et al. MDMA-assisted psychotherapy for treatment of PTSD: study design and rationale for phase 3 trials based on pooled analysis of six phase 2 randomized controlled trials. Psychopharmacol. (Berl.) 236 , 2735–2745 (2019).
Article CAS Google Scholar
Nichols, D. E. Entactogens: how the name for a novel class of psychoactive agents originated. Front. Psychiatry 13 , 863088 (2022).
Mayer, F. P. et al. Serotonin-releasing agents with reduced off-target effects. Mol. Psychiatry 28 , 722–732 (2023).
Sandtner, W. et al. Binding mode selection determines the action of ecstasy homologs at monoamine transporters. Mol. Pharmacol. 89 , 165 (2016).
Sáez-Briones, P. & Hernández, A. MDMA (3,4-methylenedioxymethamphetamine) analogues as tools to characterize MDMA-like effects: an approach to understand entactogen pharmacology. Curr. Neuropharmacol. 11 , 521–534 (2013).
Feduccia, A. A. & Mithoefer, M. C. MDMA-assisted psychotherapy for PTSD: are memory reconsolidation and fear extinction underlying mechanisms? Prog. Neuropsychopharmacol. Biol. Psychiatry 84 , 221–228 (2018).
Kamilar-Britt, P. & Bedi, G. The prosocial effects of 3,4-methylenedioxymethamphetamine (MDMA): controlled studies in humans and laboratory animals. Neurosci. Biobehav. Rev. 57 , 433–446 (2015).
Vizeli, P. et al. Effects of 3,4-methylenedioxymethamphetamine on conditioned fear extinction and retention in a crossover study in healthy subjects. Front. Pharm. 13 , 906639 (2022).
Maples-Keller, J. L. et al. A randomized controlled trial of 3,4-methylenedioxymethamphetamine (MDMA) and fear extinction retention in healthy adults. J. Psychopharmacol. 36 , 368–377 (2022).
Hysek, C. M. et al. MDMA enhances emotional empathy and prosocial behavior. Soc. Cogn. Affect. Neurosci. 9 , 1645–1652 (2014).
Brooks Holliday, S. et al. The association between discrimination and PTSD in African Americans: exploring the role of gender. Ethn. Health 25 , 717–731 (2020).
Goldstein, R. B. et al. The epidemiology of DSM-5 posttraumatic stress disorder in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. Soc. Psychiatry Psychiatr. Epidemiol. 51 , 1137–1148 (2016).
Zimmerman, M., Benjamin, I. & Seijas-Rodriguez, C. Psychiatric diagnoses among transgender and gender diverse patients compared to cisgender patients. J. Clin. Psychiatry 83 , 21m14062 (2022).
Herman, J. L. Complex PTSD: a syndrome in survivors of prolonged and repeated trauma. J. Trauma Stress 5 , 377–391 (1992).
Schein, J. et al. Prevalence of post-traumatic stress disorder in the United States: a systematic literature review. Curr. Med Res. Opin. 37 , 2151–2161 (2021).
Lewis-Schroeder, N. F. et al. Conceptualization, assessment, and treatment of traumatic stress in first responders: a review of critical issues. Harv. Rev. Psychiatry 26 , 216–227 (2018).
Williams, C. P., Senft Everson, N., Shelburne, N. & Norton, W. E. Demographic and health behavior factors associated with clinical trial invitation and participation in the United States. JAMA Netw. Open 4 , e2127792 (2021).
Ching, T. H. et al. MDMA-assisted therapy for posttraumatic stress disorder: a pooled analysis of ethnoracial differences in efficacy and safety from two phase 2 open-label lead-in trials and a phase 3 randomized, blinded placebo-controlled trial. J. Psychopharmacol. 36 , 974–986 (2022).
Schnurr, P. P. et al. Comparison of prolonged exposure vs cognitive processing therapy for treatment of posttraumatic stress disorder among US veterans: a randomized clinical trial. JAMA Netw. Open 5 , e2136921 (2022).
Jerome, L. et al. Long-term follow-up outcomes of MDMA-assisted psychotherapy for treatment of PTSD: a longitudinal pooled analysis of six phase 2 trials. Psychopharmacol. (Berl.) 237 , 2485–2497 (2020).
Lester, S. J. et al. Cardiovascular effects of 3,4-methylenedioxymethamphetamine: a double-blind, placebo-controlled trial. Ann. Intern. Med. 133 , 969–973 (2000).
Vizeli, P. & Liechti, M. E. Safety pharmacology of acute MDMA administration in healthy subjects. J. Psychopharmacol. 31 , 576–588 (2017).
Nicholas, C. R. et al. The effects of MDMA-assisted therapy on alcohol and substance use in a phase 3 trial for treatment of severe PTSD. Drug Alcohol Depend. 233 , 109356 (2022).
Feduccia, A. A. et al. Breakthrough for trauma treatment: safety and efficacy of MDMA-assisted psychotherapy compared to paroxetine and sertraline. Front. Psychiatry 10 , 650 (2019).
US Department of Veteran Affairs. PTSD: National Center for PTSD. Clinician’s guide to medications for PTSD ( https://www.ptsd.va.gov/professional/treat/txessentials/clinician_guide_meds.asp ).
Schenberg, E. E. Who is blind in psychedelic research? Letter to the editor regarding: blinding and expectancy confounds in psychedelic randomized controlled trials. Expert Rev. Clin. Pharm. 14 , 1317–1319 (2021).
Doblin, R. A clinical plan for MDMA (ecstasy) in the treatment of posttraumatic stress disorder (PTSD): partnering with the FDA. J. Psychoact. Drugs 34 , 185–194 (2002).
Weathers, F. W. et al. The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) https://www.ptsd.va.gov/ (2013).
Weathers, F. W. et al. The clinician administered PTSD scale for DSM-5 (CAPS-5): development and initial psychometric evaluation in military veterans. Psychol. Assess. 30 , 383–395 (2018).
Targum, S. D., Daly, E., Fedgchin, M., Cooper, K. & Singh, J. B. Comparability of blinded remote and site-based assessments of response to adjunctive esketamine or placebo nasal spray in patients with treatment resistant depression. J. Psychiatr. Res 111 , 68–73 (2019).
Download references
Acknowledgements
The authors thank all of the participants and their support networks. See the Supplementary Information for acknowledgments concerning study collaborators, including all members of the MAPP2 Study Collaborator Group.
This study was funded by Multidisciplinary Association for Psychedelic Studies (MAPS) with support from the Steven and Alexandra Cohen Foundation and organized by MAPS Public Benefit Corporation (PBC). MAPS PBC was responsible for overseeing the collection, analysis and interpretation of the data. Medical writing assistance was provided by J. Carpenter and M. Yochum of BOLDSCIENCE, funded by MAPS PBC.
Author information
A list of members and their affiliations appears in the Supplementary Information.
Authors and Affiliations
Neuroscape, Department of Neurology, University of California, San Francisco, San Francisco, CA, USA
Jennifer M. Mitchell
Department of Psychiatry and Behavioral Sciences, University of California, San Francisco, San Francisco, CA, USA
Jennifer M. Mitchell & Sylvestre Quevedo
Department of Veterans Affairs, Research Service, San Francisco VA Medical Center, San Francisco, CA, USA
Aguazul-Bluewater, Inc., Boulder, CO, USA
Marcela Ot’alora G.
Boston University School of Medicine, Boston, MA, USA
Bessel van der Kolk
Wholeness Center, Fort Collins, CO, USA
Scott Shannon
Department of Psychiatry, New York University Grossman School of Medicine, New York, NY, USA
Michael Bogenschutz
Zen Therapeutic Solutions, Mt. Pleasant, SC, USA
Yevgeniy Gelfand
Nautilus Sanctuary, New York, NY, USA
Casey Paleos
Department of Family Medicine and Community Health, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA
Christopher R. Nicholas
San Francisco Insight and Integration Center, San Francisco, CA, USA
Sylvestre Quevedo
New School Research, LLC, Los Angeles, CA, USA
Brooke Balliett
MAPS Public Benefit Corporation, San Jose, CA, USA
Scott Hamilton, Charlotte Harrison & Berra Yazar-Klosinski
Medical University of South Carolina, Charleston, SC, USA
Michael Mithoefer
Kleiman Consulting and Psychological Services, PC, Ivyland, PA, USA
Sarah Kleiman
KPG Psychological Services, LLC, Brunswick, ME, USA
Kelly Parker-Guilbert
University of Haifa, Haifa, Israel
Keren Tzarfaty
MAPS Israel, Hod Hasharon, Israel
Tulip Medical Consulting, LLC, Port Townsend, WA, USA
Alberdina de Boer
Multidisciplinary Association for Psychedelic Studies (MAPS), San Jose, CA, USA
Rick Doblin
You can also search for this author in PubMed Google Scholar
- Charlotte Harrison
- & Berra Yazar-Klosinski
Contributions
S.H., B.Y.-K., C.H. and A.d.B. contributed to data analysis; these authors and J.M.M. directly accessed and collectively verified the underlying data. All authors had full access to the trial data, contributed to the interpretation of the data and contributed to writing the manuscript. The authors attest to the accuracy and completeness of the reported data, accept responsibility to submit for publication and confirm that the trial conformed to the protocol and the statistical analysis plan (available via Nature Medicine ).
Corresponding author
Correspondence to Jennifer M. Mitchell .
Ethics declarations
Competing interests.
J.M.M. has received research support from MAPS; grants/contracts from the Veterans Administration (Merit Award) and the FDA (Research Award); has received royalties/licenses from UCLA (for a patent licensed to UCSF for cell screening); has received payment/honoraria from Stanford (for lecturing to undergraduate students) and Johns Hopkins (for presenting grand rounds); has a patent licensed to UCSF for cell screening; has been a reviewer for NIAAA CTN; has been a member of CA DOJ RAP; and has been a grant reviewer for the Australian Medical Research Council. M.O.G.: Aguazul-Bluewater, Inc has received research support from MAPS PBC and payments from Cybin (training and consultation), from Horizons Conference and from Naropa University. B.v.d.K. has received royalties from Penguin Random House (book, The Body Keeps the Score ) and Guilford Press (book, Traumatic Stress ); has received consulting fees from Meadows Hospital (Wickenburg, Arizona); and has received payment/honoraria from PESI. B.v.d.K. is also the president of the Trauma Research Foundation. S.S. has received grants/contracts from MAPS (research support) and MindMed (research support); has received royalties/licenses from Academic Press and Norton Publishing (professional books); has received honoraria from Scripps, the Integrative Psychiatric Institute and the Institute of Functional Medicine (lectures and presentations); is member of the Maya Health Advisory Board; and previously served as CEO of the Board of Psychedelic Medicine and Therapies. M.B. has received grant support to his institution for the current study from MAPS PBC; has received grants/contracts to his institution from Mend Medicine, Tilray Canada and the Heffter Research Institute; has been paid by AJNA Labs, Journey Colab and Bright Minds Biosciences for advisory board participation; and has received drug to his institution from Tilray Canada for an NIH-funded trial. Y.G. anticipates support from MAPS PBC for congress attendance in the future and has stock in MindMed. C.P. has received grants/contracts (research support) and consulting fees from MAPS PBC (training and supervision/consultation). C.R.N. has received grants/contracts fromm MAPS PBC (research studies); has received payment/honoraria from MAPS PBC and MindMed (training and educational events); has received meeting/travel support from MAPS PBC; and has received funding for contract work as an MDMA-assisted therapy trainer. S.Q. has received grants/contract support for research from MAPS. B.B. has received support for the present study from New School Research and the California Center for Psychedelic Therapy; has received consulting fees from MAPS PBC (training and supervision/consultation); has received payment/honoraria from the Integrative Psychiatric Institute, the California Association of Marriage and Family Therapists, the Los Angeles County Psychological Society and the Palm Springs Art Museum (lectures/speaking events); has received support for meetings/travel from MAPS PBC; and is a trainer representative for the MAPS PBC Commercial Advisory Committee and supervisor and coordinator of the Zendo Project (psychedelic harm reduction). S.H. is an employee of MAPS PBC. M.M. has received support for the present research from MAPS PBC (independent contractor) and is a member of the Awakn Life Sciences Scientific Advisory Board; has received royalties from PESI (video sales of presentations); has received consulting fees from MAPS PBC; has received payment from PESI (speaking) and the California Institute of Integral Studies (training workshops); has received honoraria from Harvard Medical School, Sounds True, the Integrative Psychiatry Institute and Vital (speaking); has received support from MAPS PBC for attending meetings/travel; and owns stock in Awakn Life Sciences. S.K. has received grants/contracts/consulting fees from MAPS PBC, pharmaceutical companies, VA hospitals and university research groups for providing supervision and training to psychodiagnostic assessors on a variety of research studies. K.P.-G. has received consulting fees from MAPS PBC for training assessors in psychodiagnostic assessment on PTSD treatment trials and from other research studies/groups conducting similar work. K.T. has received payment/honoraria from MAPS US (education and study activities in Israel), MAPS PBC (senior trainer and supervisor therapist) and MAPS Israel (co-founder and CEO) and meeting/travel support from MAPS PBC. C.H. is a MAPS PBC employee; has received consulting fees from Cybin (unrelated molecule and indication in the psychedelics field); and has received meeting/travel support from MAPS PBC. A.d.B. was previously an employee of MAPS PBC; has received consulting fees (to Tulip Medical Consulting); has received meeting travel/support from MAPS PBC; and served as CMO (while previously an employee of MAPS PBC) and attended Data Safety Monitoring Board meetings without any voting rights. R.D. is the founder and president (salaried employee) of MAPS and is a member of the Board of MAPS and MAPS PBC. B.Y.-K. is an employee of MAPS PBC, was previously an employee of MAPS and has received support from MAPS PBC and MAPS for attending meetings/travel.
Peer review
Peer review information.
Nature Medicine thanks Matthias Liechti, Alimu Dayimu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary handling editor: Jerome Staal, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information.
CONSORT checklist, MAPP2 study collaborators, Supplementary Methods, Supplementary Figs. 1 and 2, Supplementary Tables 1–9 and Supplementary References.
Reporting Summary
Source data fig. 2.
Statistical source data.
Source Data Fig. 3
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Mitchell, J.M., Ot’alora G., M., van der Kolk, B. et al. MDMA-assisted therapy for moderate to severe PTSD: a randomized, placebo-controlled phase 3 trial. Nat Med 29 , 2473–2480 (2023). https://doi.org/10.1038/s41591-023-02565-4
Download citation
Received : 02 June 2023
Accepted : 24 August 2023
Published : 14 September 2023
Issue Date : October 2023
DOI : https://doi.org/10.1038/s41591-023-02565-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Optimizing real-world benefit and risk of new psychedelic medications: the need for innovative postmarket surveillance.
- Joshua C. Black
- Andrew A. Monte
- Richard C. Dart
Nature Mental Health (2024)
Side-effects of mdma-assisted psychotherapy: a systematic review and meta-analysis
- Julia Colcott
- Alexandre A. Guerin
- Gillinder Bedi
Neuropsychopharmacology (2024)
Newly identified structures of trace-amine associated receptor-1 (TAAR1) will aid discovery of next generation neuropsychiatric drugs
- Pramod C. Nair
- Britto Shajan
- Tarun Bastiampillai
Molecular Psychiatry (2024)
Nichtansprechen auf Psychotherapie: Konzepte, Problemstellungen, Zuweisungsoptionen
- Jürgen Hoyer
Der Nervenarzt (2024)
The entactogen 3,4-methylenedioxymethamphetamine (MDMA; ecstasy) as a treatment aid in psychotherapy and its safety concerns
- Brian A. Baldo
Archives of Toxicology (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

Sleep impairments in refugees diagnosed with post-traumatic stress disorder: a polysomnographic and self-report study
Affiliations.
- 1 Competence Centre for Transcultural Psychiatry (CTP), Mental Health Centre, Ballerup, Copenhagen University Hospital - Mental Health Services CPH, Copenhagen, Denmark.
- 2 Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark.
- 3 Department Bispebjerg-Gentofte, Mental Health Centre Copenhagen, Copenhagen, Denmark.
- 4 Danish Centre for Sleep Medicine (DCSM), Copenhagen University Hospital - Rigshospitalet-Glostrup, Copenhagen, Denmark.
- PMID: 36971225
- PMCID: PMC10044313
- DOI: 10.1080/20008066.2023.2185943
Abstract in English, Spanish, Chinese
Background: Post-traumatic stress disorder (PTSD) is the clinical manifestation of traumatic events and is associated with sleep disturbances. Sleep disturbances, if left untreated, may perpetuate or even worsen symptoms of PTSD. Previous studies of other PTSD populations show a higher incidence of sleep impairments and sleep disorders compared to healthy controls (HCs); however, this has never been investigated in trauma-affected refugees diagnosed with PTSD. Objectives: To examine subjective sleep quality, measure sleep architecture, and identify latent sleep disorders in refugees diagnosed with PTSD compared to HCs. Method: This comparative study included 20 trauma-affected refugees diagnosed with PTSD and 20 HC matched on age, sex, and body mass index. All participants completed self-report questionnaires assessing sleep quality, insomnia severity, and disturbing nocturnal behaviour, and all took part in a one-night polysomnography (PSG) assessment. Results: Patients reported significantly poorer subjective sleep quality, sleep latency, sleep duration, and sleep efficiency compared to HCs. Subjective reports on hours spent in bed were not significantly different between patients and HCs. Patients reported significantly higher nightmare frequency and severity compared to HCs. PSG measures showed that patients had significantly reduced sleep efficiency, more awakenings, and longer REM sleep latency, and spent more time awake, whereas there was no significant differences regarding total time in bed, total sleep time, or sleep latency. The prevalence of sleep disorders was equal between groups. Conclusions: The study identified significant impairments in several sleep domains, with a preponderance of disturbed regulation of sleep resulting in awakenings. These results indicate a need for more focus on hyperarousal and nightmares as key elements of disturbed sleep in PTSD. Furthermore, the study identified a discrepancy between subjective and objective measures concerning total sleep time, raising questions regarding the causes of 'sleep state misperception'. Trial registration: ClinicalTrials.gov identifier: NCT03535636 .. Trial registration: Sleep Impairments in Refugees Diagnosed with PTSD (PSG-PTSD). URL: https://clinicaltrials.gov/ct2/show/NCT03535636. ClinicalTrials.gov NCT03535636 . Date of registration: 24/05/2018.
Antecedentes: El trastorno de estrés postraumático (TEPT) es la manifestación clínica de los eventos traumáticos y está asociado a deterioro del sueño. El deterioro del sueño, si no es tratado, puede perpetuar o, incluso, empeorar los síntomas del TEPT. Estudios previos realizados en otras poblaciones con TEPT muestran una incidencia más alta de deterioro del sueño y de trastornos del sueño en comparación con controles sanos. Sin embargo, esto no ha sido investigado en refugiados con diagnóstico del TEPT.
Objetivo: Evaluar la calidad subjetiva del sueño, determinar la arquitectura del sueño e identificar los trastornos latentes del sueño en refugiados con diagnóstico del TEPT y compararlos con controles sanos.
Métodos: Este estudio comparativo incluyó a veinte refugiados afectados con trauma y con diagnóstico del TEPT y a veinte controles sanos pareados por edad, sexo e índice de masa corporal (IMC). Todos los participantes respondieron preguntas de autorreporte para evaluar la calidad del sueño, severidad del insomnio y conductas perturbadoras durante el sueño; a todos los participantes se les realizó una evaluación mediante polisomnografía (PSG) durante una noche.
Resultados: Los pacientes reportaron de forma significativa una pobre calidad subjetiva de sueño, latencia del sueño, duración del sueño y eficiencia del sueño comparados con los controles sanos. Los reportes subjetivos en relación con las horas en cama no fueron significativamente distintos entre pacientes y controles sanos. Los pacientes reportaron una frecuencia y severidad más altas de pesadillas en comparación con los controles sanos. Las mediciones mediante PSG mostraron que los pacientes presentaban significativamente una menor eficiencia del sueño, más despertares nocturnos, mayor latencia del sueño REM y mayor tiempo despiertos, mientras que no se encontraron diferencias significativas en el tiempo total en cama, duración total del sueño o latencia del sueño. La prevalencia de los trastornos del sueño fue igual en ambos grupos.
Conclusiones: El estudio identificó un deterioro significativo en distintos dominios del sueño con una tendencia hacia el compromiso de la regulación del sueño, llevando a despertares nocturnos. Estos resultados muestran la necesidad de mejorar el abordaje de la hipervigilancia y las pesadillas como elementos clave en el deterioro del sueño en personas con el TEPT. Además, el estudio identificó una discrepancia entre las medidas subjetivas y objetivas del sueño en relación con el tiempo de sueño total, generando interrogantes sobre la causa de esta “percepción errónea de las etapas del sueño”.
背景: 创伤后应激障碍(PTSD)是创伤事件的临床表现,与睡眠障碍相关。 如果不及时治疗,睡眠障碍可能会持续存在,甚至会加重 PTSD 的症状。 先前对其他 PTSD 人群的研究表明,与健康对照组 (HC) 相比,睡眠受损和睡眠障碍的发生率更高,但从未在被诊断患有 PTSD 的受创伤影响的难民中进行过调查。
目的: 在被诊断患有 PTSD 的难民中,对比健康对照组,考查其主观睡眠质量、测量睡眠结构并识别潜在的睡眠障碍。
方法: 这项比较研究包括 20 名被诊断患有 PTSD 的受创伤影响的难民和 20 名年龄、性别和 BMI 匹配的 HC。 所有参与者都完成了评估睡眠质量、失眠严重程度和困扰的夜间行为的自我报告问卷,并且都参加了一晚多导睡眠图 (PSG) 评估。
结果: 与 HC 相比,患者报告的主观睡眠质量、睡眠潜伏期、睡眠持续时间和睡眠效率显著较差。 关于卧床时间的主观报告在患者和 HC 之间没有显著差异。 与 HC 相比,患者报告的噩梦频率和严重程度显著更高。 PSG 测量显示,患者的睡眠效率显著降低、觉醒次数增多、REM 睡眠潜伏期延长以及清醒时间增加,而在床上的总时间、总睡眠时间或睡眠潜伏期方面没有显著差异。 睡眠障碍的流行率在各组之间是相等的。
结论: 该研究确定了几个睡眠领域的显著受损,其中主要是睡眠调节紊乱导致觉醒。 这些结果表明需要更多地关注高唤起和噩梦作为 PTSD 中睡眠障碍的关键因素。 此外,该研究发现了引发“睡眠阶段误解”的关于总睡眠时间的主观和客观测量之间的差异。
Keywords: Polisomnografía; Polysomnography; calidad del sueño; post-traumatic stress disorder; refugees; refugiados; sleep disturbances; sleep quality; trastorno de estrés postraumático; trastornos del sueño; trauma; 创伤; 创伤后应激障碍; 多导睡眠图; 睡眠质量; 睡眠障碍; 难民.
Plain language summary
This is the first study assessing sleep impairments and sleep disorders in refugees diagnosed with post-traumatic stress disorder (PTSD) compared to healthy controls.The main finding is that both subjectively and objectively measured sleep is disrupted in refugees diagnosed with PTSD compared to healthy controls.The results suggest that these disturbances of sleep are significant targets in treatment of PTSD and stress the importance of focusing on treatment of sleep disturbances in PTSD.
Publication types
- Clinical Trial
- Research Support, Non-U.S. Gov't
- Self Report
- Sleep Initiation and Maintenance Disorders* / complications
- Sleep Wake Disorders* / epidemiology
- Sleep Wake Disorders* / etiology
- Stress Disorders, Post-Traumatic* / complications
- Stress Disorders, Post-Traumatic* / epidemiology
Associated data
- ClinicalTrials.gov/NCT03535636
Grants and funding
ORIGINAL RESEARCH article
A study on post-traumatic stress disorder and post-traumatic growth among patients infected with covid-19 in wuhan.

- 1 School of Nursing, Fujian Medical University, Research Center for Nursing Humanity, Fuzhou, Fujian, China
- 2 Department of Psychology, Army Medical University, Chongqing, China
- 3 Anesthesiology Department, The 965 Hospital of the Joint Logistic, Jilin, China
- 4 The First Affiliated Hospital of Army Medical University, Chongqing, China
Objective: The purpose of this study is to assess the physical and psychological conditions of hospitalized patients who were infected with COVID-19 in Wuhan, China, including post-traumatic stress disorder (PTSD) and post-traumatic growth (PTG) scores and predictors.
Methods: The test group consisted of 102 hospitalized patients diagnosed with COVID-19 in Wuhan between March 4, 2020 and April 5, 2020, whereas the control group comprised 168 healthy study participants. Relevant information of the study participants was obtained using online questionnaires, covering five aspects—general information, physical state, emotional state, PTSD, and PTG.
Results: In Wuhan, 37.3% of COVID-19-diagnosed hospitalized patients exhibited hyper-arousal symptoms of PTSD. This percentage is significantly higher than the 13.1% observed in the healthy population. Furthermore, the prevalence of PTG among the same group of hospitalized patients stood at 77.5%, surpassing the 66.1% rate found within the healthy population. It was determined that inconsistent sleep patterns during the hospitalization phase could be indicative of heightened vulnerability to hyperarousal symptoms of PTSD in COVID-19-diagnosed hospitalized patients. The study determined that inconsistent sleep patterns during hospitalization may be a predisposition factor that makes hospitalized patients diagnosed with covid-19 more susceptible to high arousal symptoms of post-traumatic stress disorder. Conversely, COVID-19-diagnosed hospitalized patients who maintained a tranquil demeanor and exhibited positive emotional perceptions during their hospitalization displayed reduced susceptibility to these PTSD symptoms. Factors such as possession of a bachelor’s degree, history of severe acute respiratory syndrome (SARS) infection, and poor sleep patterns were identified as predictors elevating the risk of PTG. Whereas, a sentiment of happiness and consistent positive emotional perception during hospitalization were predictors of PTG. Intriguingly, a direct correlation was established between hyper-arousal symptoms of PTSD and PTG.
Conclusion: Although the outbreak of COVID-19 has badly affected the physical and psychological well-being of patients, it has greatly enhanced their PTG.
1 Introduction
On January 30, 2020, the World Health Organization (WHO) recognized the COVID-19 outbreak as a public health emergency of an international magnitude ( Li et al., 2020 ; Wang et al., 2020 ). By August 9, 2020, there were 19,462,112 globally confirmed COVID-19 cases.
Undoubtedly, COVID-19 represents a profound traumatic event, eliciting acute traumatic responses amongst patients. Numerous patients manifested various physical and psychological symptoms during the pandemic, notably sleep disturbances, anxiety, and depression ( Hong et al., 2009 ; Lee et al., 2018 ). In the preliminary epicenter of the outbreak, Wuhan, in China, mandatory isolation was imposed on patients diagnosed with COVID-19. The ambiguity and apprehension associated with this novel virus invariably resulted in enhanced psychological strain and emotional turbulence among these patients, culminating, in grave instances, into post-traumatic stress disorder (PTSD).
PTSD represents a multifaceted disorder, commonly precipitating pronounced psychological distress and impeding normal social interactions. Compared to those with standard traumatic responses, patients suffering from PTSD exhibit intensified conditions marked by intrusive thoughts, evasive behavior, emotional volatility, feelings of detachment, and heightened threat alertness ( Sareen et al., 2005 ). However, PTSD does not invariably follow traumatic events. Merely a minuscule fraction of those undergoing traumatic experiences eventually manifest PTSD. Sayed et al. stratified PTSD risk factors into pre-trauma, pri-trauma, and post-trauma phases ( Sayed et al., 2015 ). Drawing from extant literature, our study highlighted pre-trauma predictors linked to PTSD, encompassing gender, age, educational background, and antecedent trauma exposure ( Ozer et al., 2003 ; Kessler et al., 2005 ; Barnett et al., 2006 ; McGrath et al., 2015 ). Pri-trauma predictors associated with PTSD predominantly focus on the cognitive and biological trauma processing of the patients, their physiological stress response, trauma severity, and subjective emotional responses ( Carlier et al., 1997 ; Breslau et al., 2005 ). Post-trauma PTSD involves psychological and societal dimensions, such as optimism, cognitive adaptability, proactive coping mechanisms, and the degree of social support.
While the COVID-19 outbreak undeniably impinged on the physical and mental health of its victims, it might paradoxically engender positive ramifications. As empirical studies suggest, traumatic incidents can catalyze post-traumatic growth (PTG) among affected patients ( Arpawong et al., 2013 ). PTG denotes the beneficial psychological changes experienced by individuals post traumatic incidents ( Tedeschi and Calhoun, 1996 ). Contemporary academic discourse, pivoting toward positive psychology, increasingly emphasizes the benefits individuals accrue from adversities. PTG, a prevalent aftermath of traumatic events, often leads to more pervasive positive adjustments than its maladaptive counterparts ( Tedeschi and Calhoun, 2004 ). Recent studies demonstrated that PTG predictors exhibit significant overlaps with those of PTSD ( Dekel et al., 2011 ; Jin et al., 2014 ; Tomita et al., 2017 ). Another large-sample study underscored a weak positive correlation between PTSD and PTG ( Klosky et al., 2014 ).
Currently, comprehensive insights into the psychological ramifications of COVID-19 among hospitalized patients in Wuhan remain elusive. This study endeavors to elucidate the physical and mental status of such patients, concentrating on the quantifiable metrics and predictors of PTSD and PTG.
2 Study participants and methods
2.1 study participants.
The present study was of a cross-sectional design. A convenience sampling method was used. This study encompassed a sample of 102 hospitalized patients from Wuhan City, diagnosed with COVID-19 between March 4, 2020, and April 5, 2020, forming the test group. The criteria for inclusion were as follows: (1) Participants willingly engaged in the study and provided their signed informed consent. (2) Participants satisfied the COVID-19 diagnostic criteria ( Diagnosis and treatment, 2020 ). (3) between the 3rd and 10th day of hospitalization. (4) Participants demonstrated the capability to read and exhibited a reasonable comprehension level. (5) Participants had the proficiency to complete the online questionnaires, either directly through electronic devices or with external assistance.
A control group was constituted, comprising 168 healthy individuals from WeChat (a widely used social application in China with over 1 billion active users). Their inclusion criteria comprised: (1) Participants no satisfied the COVID-19 diagnostic criteria ( Diagnosis and treatment, 2020 ). (2) Participants provided an informed consent; (3) Participants demonstrated the capability to read and exhibited a reasonable comprehension level. (4) Participants had the proficiency to complete the online questionnaires.
Patients diagnosed with COVID-19 were recruited from Huoshenshan Hospital and Taikang Hospital, which were the largest hospitals admitting COVID-19 inpatients in Wuhan during the COVID-19 pandemic. The distribution of patients’ age, gender, and severity of disease was consistent with the overall data trend published by the National Health Commission of the People’s Republic of China. The age of healthy individuals ranged from 18 to 89 years and covered a wide range of age groups. There was no difference in gender distribution among healthy individuals (including 88 males and 80 females). In addition, healthy individuals reported all types of occupations, including farmers, soldiers, students, doctors, nurses, freelancers, police, retired workers, civil servants, sales, teachers, and others. Thus, the current sample is representative of the whole population.
2.2 Survey tools
In the midst of the critical epidemic response phase in Wuhan, we designed a questionnaire considering the operational feasibility and the circumstances of isolated, hospitalized patients diagnosed with COVID-19. This included taking into account their disease severity, emotional state, stress responses, and the acceptable duration for responding. Instead of adopting internationally standardized psychological test questionnaires with an exhaustive list of questions, we utilized a tailored assessment and interview questionnaire focusing on the psychological health of patients with the novel coronavirus. This tool was meticulously crafted through the concerted efforts of Professor Dai Qin’s team from the Psychological Survey and Research Division of the Army Medical University. Its development drew upon existing validated questionnaires from both domestic and international sources. Strategies such as literature review, expert consultations, and brainstorming sessions were employed. Additionally, we integrated insights from contemporary international and Chinese literature and aligned with the “Diagnosis and Treatment Scheme for Novel Coronavirus-Infected Pneumonia (Trial 5th Edition)” ( Diagnosis and treatment, 2020 ).
Our questionnaire encompasses four domains: Pre-traumatic Information, Pri-traumatic Information, and hyper-arousal symptom of PTSD and PTG.
2.2.1 Pre-traumatic information
This section captures data such as gender, age, marital status, educational level, and history of SARS infection.
2.2.2 Pri-trauma information
This section was collected from two dimensions: Physical State and Emotional State.
Physical State: Here, participants provide details about their daily routines, dietary patterns, sleep quality, and disease severity. The Physical State was rated using a “yes” or “no” response. Cronbach’s alpha coefficient for Physical State was 0.617, and the KMO coefficient was 0.702, accounting for 56.2% of the total variance.
Emotional State: This part assesses emotional fluctuations across six dimensions—serenity, anxiety, anger, melancholy, fear, and happiness. The degree of emotional reaction was examined using a five-point Likert scale (how serene [anxious, angry, fearful, melancholy, and happy] you feel following your hospitalization for COVID-19): 1, no; 2, mild; 3, moderate; 4, severe; 5, extreme. The Cronbach’s alpha was 0.82, and the KMO coefficient was 0.797, accounting for 64.9% of the total variance.
We utilized the PTSD Symptom Checklist, a concise tool designed to gauge post-traumatic stress symptoms and promptly identify individuals predisposed to PTSD ( Conybeare et al., 2012 ). The 17-item checklist allows respondents to rate their symptoms on a scale from 1 (indicating no specific symptom) to 5 (indicating an extremely severe symptom). Higher aggregate scores denote an elevated propensity for PTSD. This checklist has three dimensions: populations re-experiencing symptoms, populations with persistent avoidance/numbing symptoms, and populations with hyper-arousal symptoms. Our survey primarily targeted the PTSD assessment for populations with hyperarousal symptoms. The total score was calculated by summing the scores of each item (items 13–17); any item with a score ≥ 3 or any 2/5 hyper-arousal items among items 13–17 were considered positive symptoms. Cronbach’s alpha coefficient for hyper-arousal symptoms was 0.843.
We deployed the PTG Scale, which is used to measure the beneficial psychological improvements of persons who have endured severely traumatic life events or situations ( Tedeschi and Calhoun, 1996 ). Cronbach’s alpha coefficient for PTG was 0.90. This 21-item tool has five distinct dimensions: personal strength, relationships with others, newfound possibilities, spiritual transformation, and life appreciation. Responses range from 0 (no experienced change post-trauma) to 5 (significant post-traumatic change). PTG total scores vary from 0 to 105. Elevated aggregate scores indicate enhanced PTG levels. A benchmark score of 63 delineated the threshold for PTG presence.
2.3 Survey methods
We surveyed both hospitalized patients diagnosed with COVID-19 (between the 3rd and 10th day of hospitalization) and healthy study participants in Wuhan from 4th March, 2020 to 5th April, 2020, approximately the first pandemic peak of the COVID-19 outbreak in Wuhan. Data collection was facilitated through an online platform. 1 Initially, we signed up for the Wenjuanxing platform and subsequently imported the questionnaire content into it. This process enabled us to obtain a link to the questionnaire. We then disseminated this link to the study participants’ mobile phones through WeChat, facilitating timely completion of the survey. Before the test began, hospitalized patients diagnosed with COVID-19 and healthy study participants were told the purpose of the measurements and read the informed consent to understand that they could refuse to answer or quit answering at any time. To eliminate duplicate submissions, this study limited each IP address to only one submission. By the end of the study, a total of 102 patients diagnosed with COVID-19 were recruited. All submissions from the test group were valid, yielding a 100% effective response rate. From the healthy study participants, 184 questionnaires were collected, of which 168 were deemed valid. This translated to an effective response rate of 91.3% for the control group.
2.4 Statistical methods
Data derived from the survey was subjected to statistical analysis using the SPSS 22.0 statistical software. A series of tests, including the two independent samples t-test, rank sum test, and chi-squared test, were applied to examine the general characteristics, physical and psychological states, post-traumatic stress reactions, and PTG levels of both the test group and the control group in Wuhan. A stratified regression analysis was specifically utilized to analyze the predictors of PTSD and PTG among the COVID-19 patient group.
3.1 Comparison of the demographics, and physical and emotional states of hospitalized patients diagnosed with COVID-19 and healthy study participants in Wuhan
In the Pre-traumatic Information analysis, there was no statistically significant difference in gender between the test and the control groups ( Table 1 ). Conversely, age, marital status and education level manifested statistically significant differences with a significance level of p < 0.05. In the Pre-traumatic Information analysis, with respect to Physical State, the prevalence of irregular daily routine was found to be 49.0% in patients diagnosed with COVID-19 compared to 29.2% in the control group, demonstrating a significant difference. Moreover, the incidence of poor sleep quality was registered at 43.1% for patients diagnosed with COVID-19 as opposed to 18.5% for the control group, with the difference being statistically significant at p < 0.05. From the Emotional State perspective, the proportion of individuals reporting a serene disposition amounted to 69.6% for patients diagnosed with COVID-19 and 87.5% for the control group, presenting a significant difference at p < 0.05. A detailed comparison of these variables between the groups can be found in Table 1 .
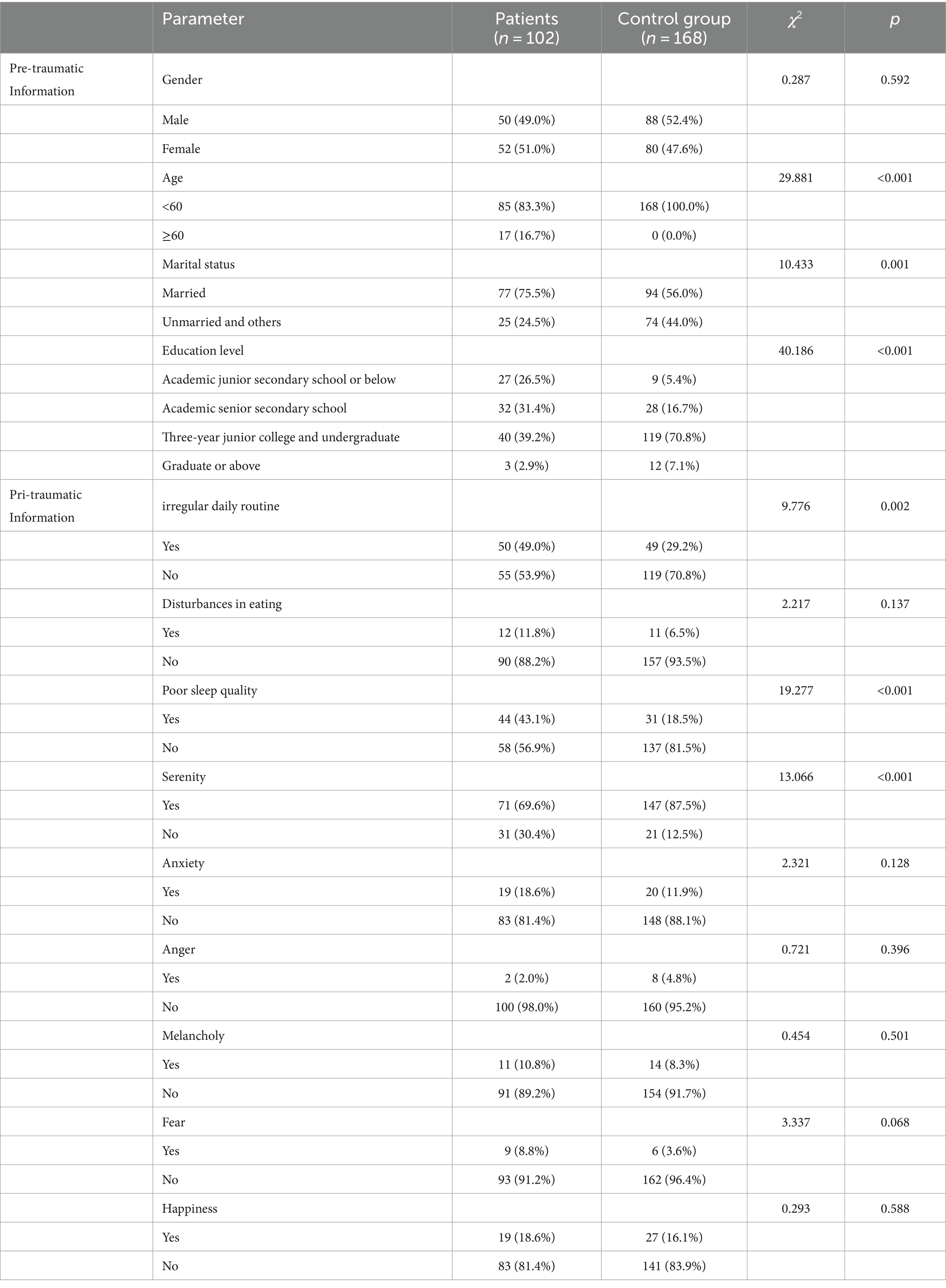
Table 1 . Comparison of the demographics, and physical and emotional states of hospitalized patients diagnosed with COVID-19 and healthy study participants in Wuhan.
3.2 Comparison of hyper-arousal symptoms of PTSD and PTG between hospitalized patients with COVID-19 and healthy study participants in Wuhan
Within Wuhan, the prevalence of hyperarousal symptoms of PTSD was observed to be 37.3% among hospitalized patients diagnosed with COVID-19 compared to 13.1% in the healthy population ( Table 2 ). Notably, this prevalence was significantly higher in the test group, as evidenced by a significance level of p < 0.05, as detailed in Table 2 . Furthermore, the incidence of PTG was recorded at 77.5% for hospitalized patients with COVID-19, in contrast to 66.1% among the healthy group within the city. Again, this rate was markedly higher in the test group, with a significant difference at p < 0.05, as displayed in Table 2 .
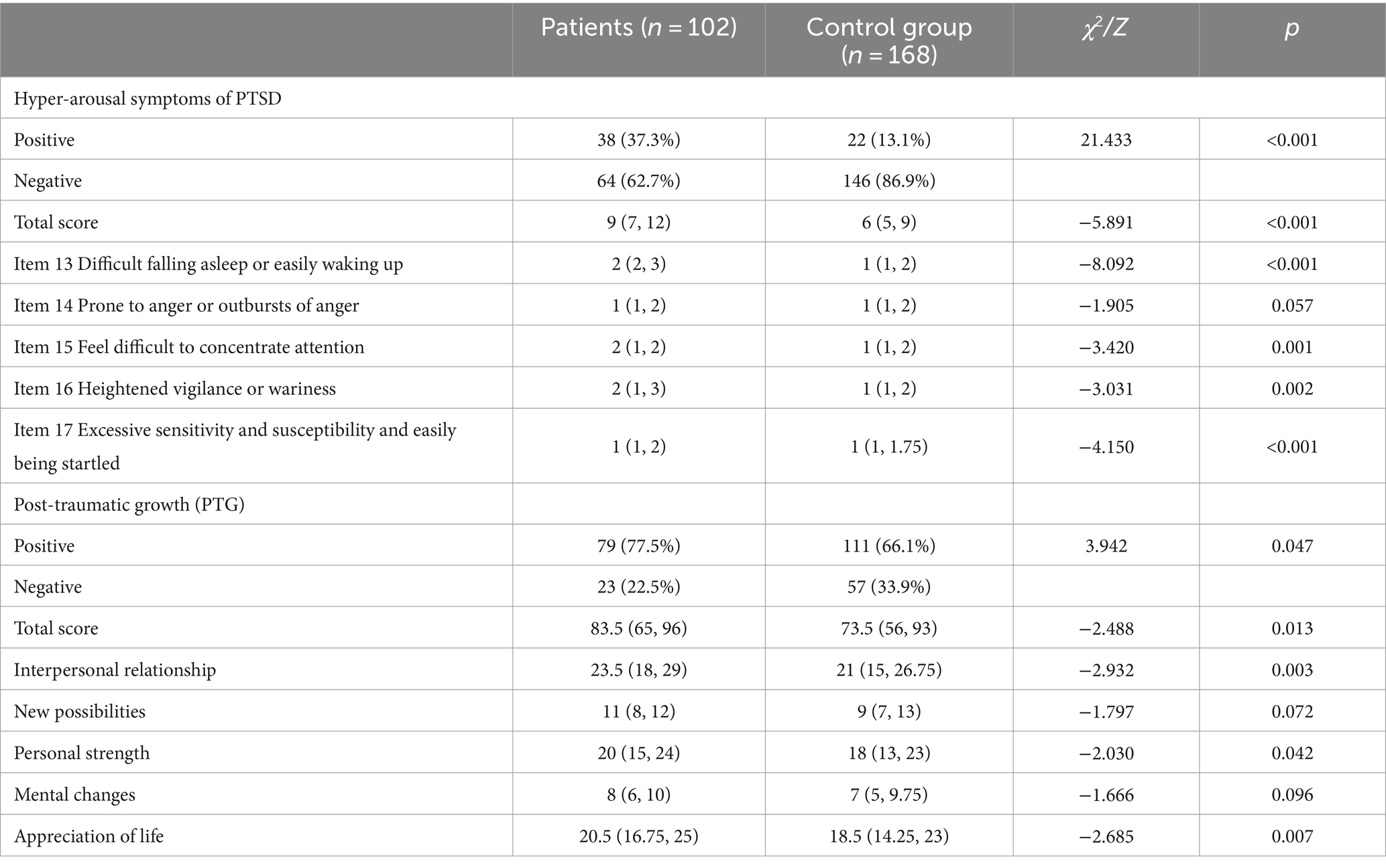
Table 2 . Comparison of hyper-arousal symptoms of PTSD and PTG between hospitalized patients with COVID-19 and healthy study participants in Wuhan.
3.3 Characteristic of the scores of hyperarousal symptoms of PTSD and PTG scores in hospitalized patients with COVID-19 in Wuhan
In Wuhan, hospitalized patients diagnosed with COVID-19 manifested significant differences in the cumulative score reflecting hyper-arousal symptoms of PTSD ( Table 3 ). These differences were linked to physical factors, specifically, the regularity of their daily routines, the quality of their sleep, and the degree of disease from COVID-9. Emotional parameters, such as sensations of serenity, anxiety, melancholy, and fear, were also influential (p < 0.05). Furthermore, patients characterized by irregular daily routines, suboptimal sleep quality, severe illness, anxiety, melancholy, and fear registered elevated scores for hyper-arousal symptoms of PTSD. Whereas patients who portrayed a sense of serenity exhibited lower scores, as displayed in Table 3 .
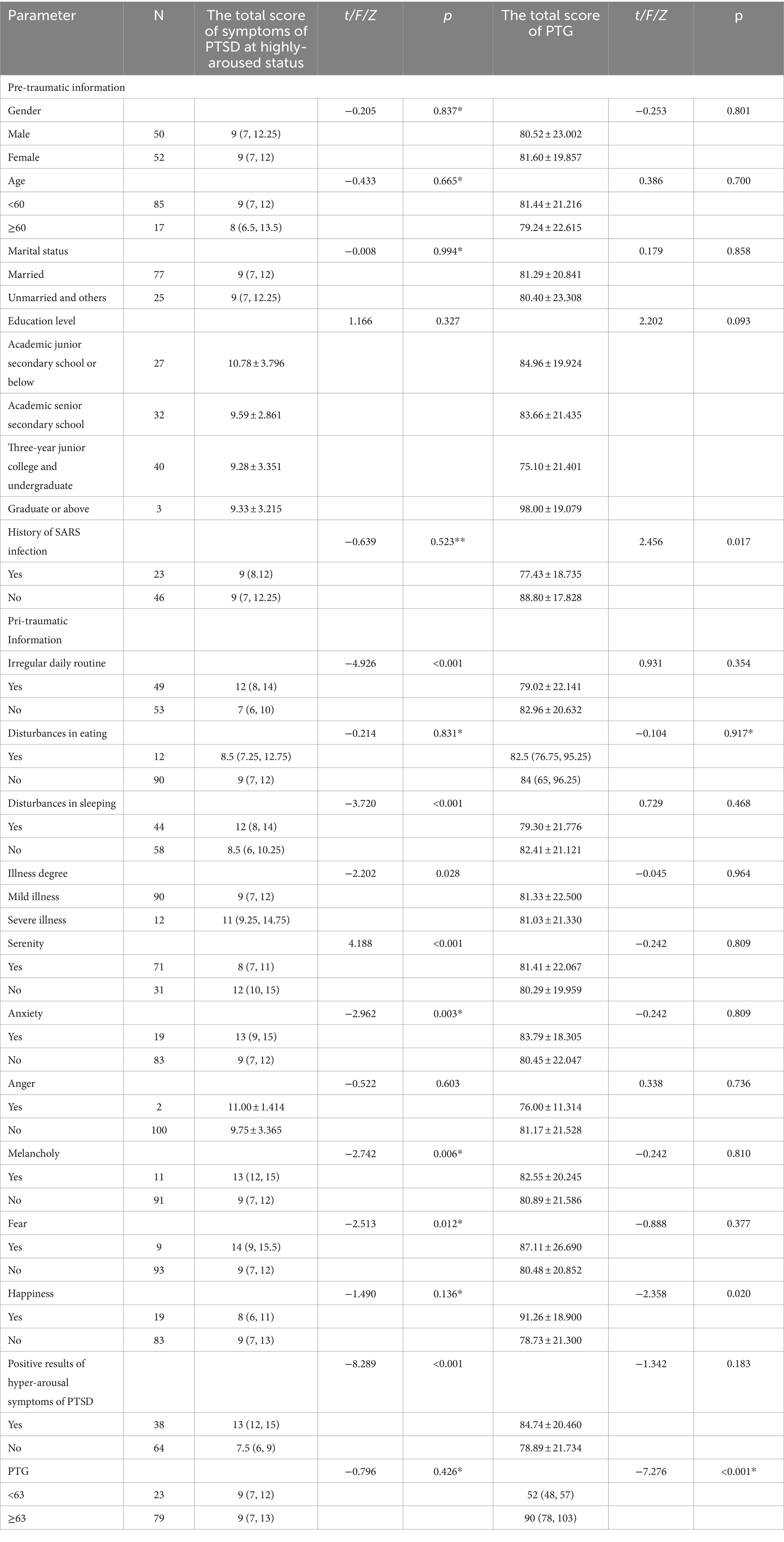
Table 3 . Characteristic of the scores of hyperarousal symptoms of PTSD and PTG scores in hospitalized patients with COVID-19 in Wuhan.
Additionally, statistically significant differences were observed in the aggregate score of PTG among hospitalized patients diagnosed with COVID-19 in Wuhan when stratified by Pre-traumatic Information attributes, in particular, those who had a history of severe acute respiratory syndrome (SARS) infection. Emotional states dimension of Pri-traumatic Information, such as the presence of happiness, also influenced these scores (p < 0.05). More specifically, patients recounting feelings of happiness in their emotional experiences demonstrated higher PTG scores. In contrast, those with a prior history of SARS reflected lower PTG scores, as displayed in Table 3 .
3.4 Analysis of predictors of PTSD in hospitalized patients diagnosed with COVID-19 in Wuhan
A stratified regression analysis was performed, featuring three tiers of stratification, with the aggregate score of hyper-arousal symptoms of PTSD serving as the dependent variable ( Table 4 and Figure 1 ). The factors considered potentially influential on the hyper-arousal symptoms of PTSD score were categorized into these three stratifications. Specifically, the initial stratification captured pre-trauma predictors, which included gender, age, marital status, educational level, and a history of SARS infection. The subsequent stratification incorporated variables encountered during the traumatic phase, namely daily routine, dietary patterns, sleep quality, disease severity, as well as emotional indicators such as serenity, anxiety, anger, melancholy, fear, and happiness. The concluding stratification was solely centered around the aggregate PTG score. Based on the results derived from this analysis, two salient variables—irregular daily routine and a sense of serenity—were integrated into the regression equation. Within this context: The pre-trauma variables were responsible for elucidating 6.8% of the variability linked to hyper-arousal symptoms of PTSD for patients diagnosed with COVID-19 in Wuhan (R 2 = −0.068). The peri-traumatic variables elucidated 28.8% of the variability (△R 2 = 0.288). The total PTG score accounted for a 1.4% explanatory power regarding the variability (△R 2 = 0.014). Obviously, peri-traumatic variables were the stronger predictors of hyper-arousal symptoms. Notably, the hyper-arousal symptoms of PTSD were observed to be elevated in patients marked by erratic daily routines (β = 0.441, p = 0.006) and attenuated in those exuding emotional serenity (β = −0.457, p = 0.004). It is imperative to highlight that the variables encompassed within the regression model provided only a fractional elucidation of the outcome, suggesting the possible influence of unexplored factors on the hyperarousal symptoms of PTSD, as visualized in Table 4 and Figure 1 .
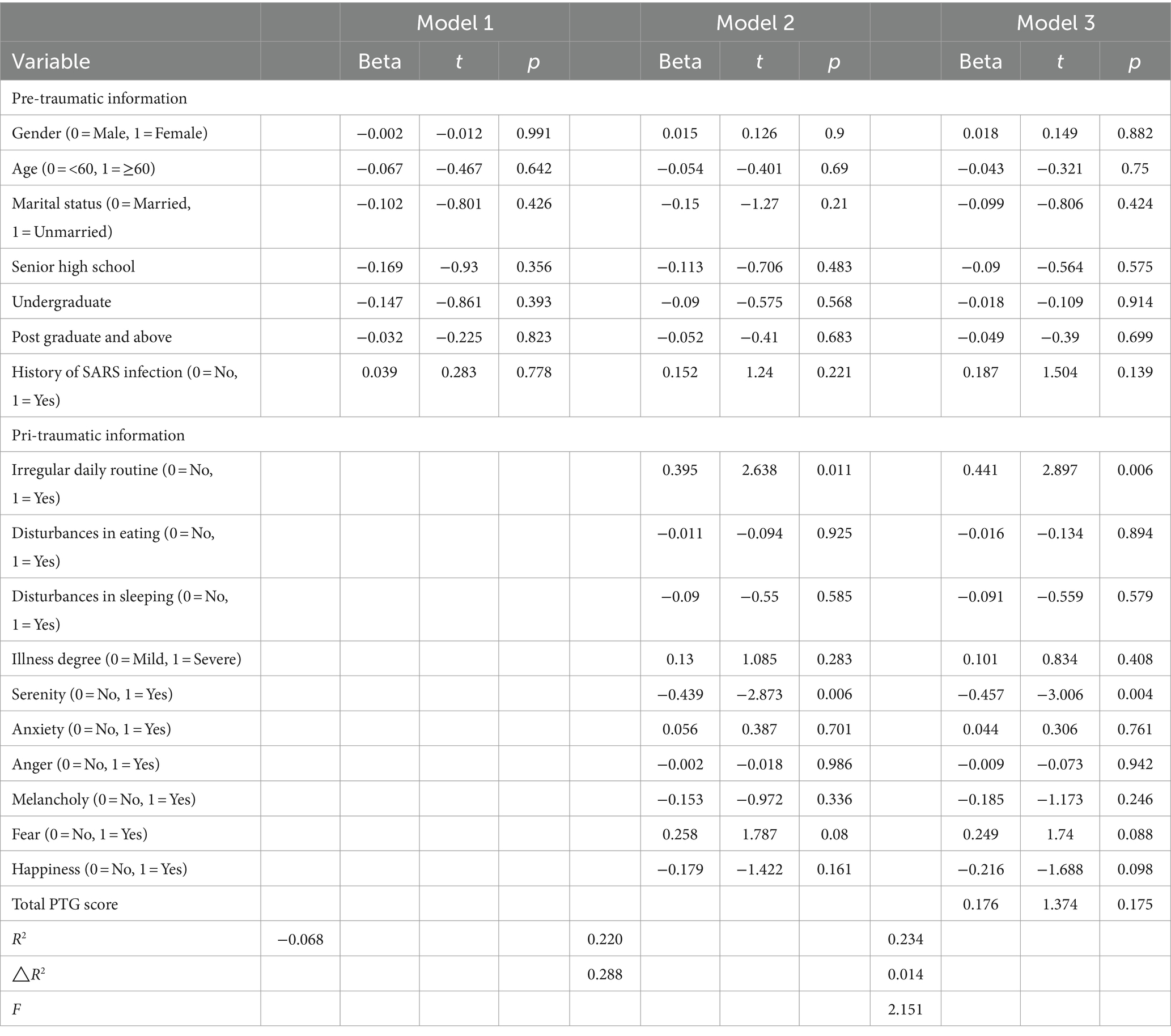
Table 4 . Predictors of PTSD in hospitalized patients diagnosed with COVID-19 in Wuhan.
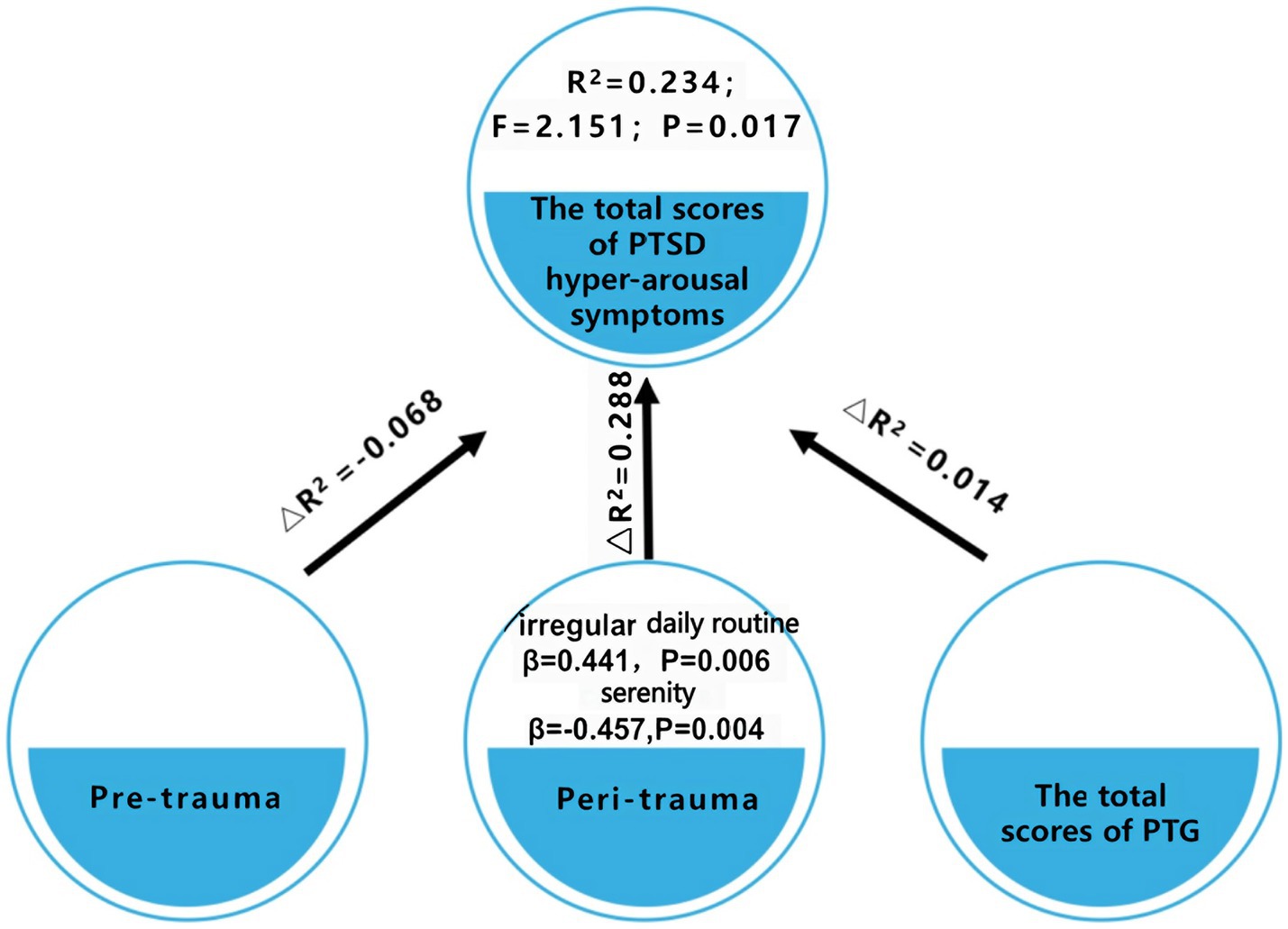
Figure 1 . Evaluation of PTSD risk factors in patients diagnosed with COVID-19 in Wuhan.
3.5 Analysis of predictors of PTG in patients diagnosed with COVID-19 in Wuhan
A stratified regression analysis was conducted, stratified into three tiers, with the total score of PTG serving as the dependent variable ( Table 5 and Figure 2 ). The factors perceived to potentially influence the PTG score were categorized into these three stratifications: The initial stratification focused on pre-trauma factors. The subsequent stratification incorporated factors pertinent during the trauma phase. The final stratification emphasized the positive rate of hyper-arousal symptoms of PTSD. Emerging from this analysis, five distinct variables—educational level, a history of SARS infection, irregular daily routines, a sensation of happiness, and the positive rate of hyper-arousal symptoms of PTSD were incorporated into the regression equation. In this context, the pre-trauma variables accounted for 13.6% of the variability in the PTG scores among patients diagnosed with COVID-19 in Wuhan (R 2 = 0.136). The factors during the trauma phase explained 3.5% of the variability in the PTG scores (△R 2 = 0.035). The positive rate of hyper-arousal symptoms of PTSD symptoms elucidated 8.4% of the variability in PTG (△R 2 = 0.084).
Table 5 . Predictors of PTG in hospitalized patients diagnosed with COVID-19 in Wuhan.
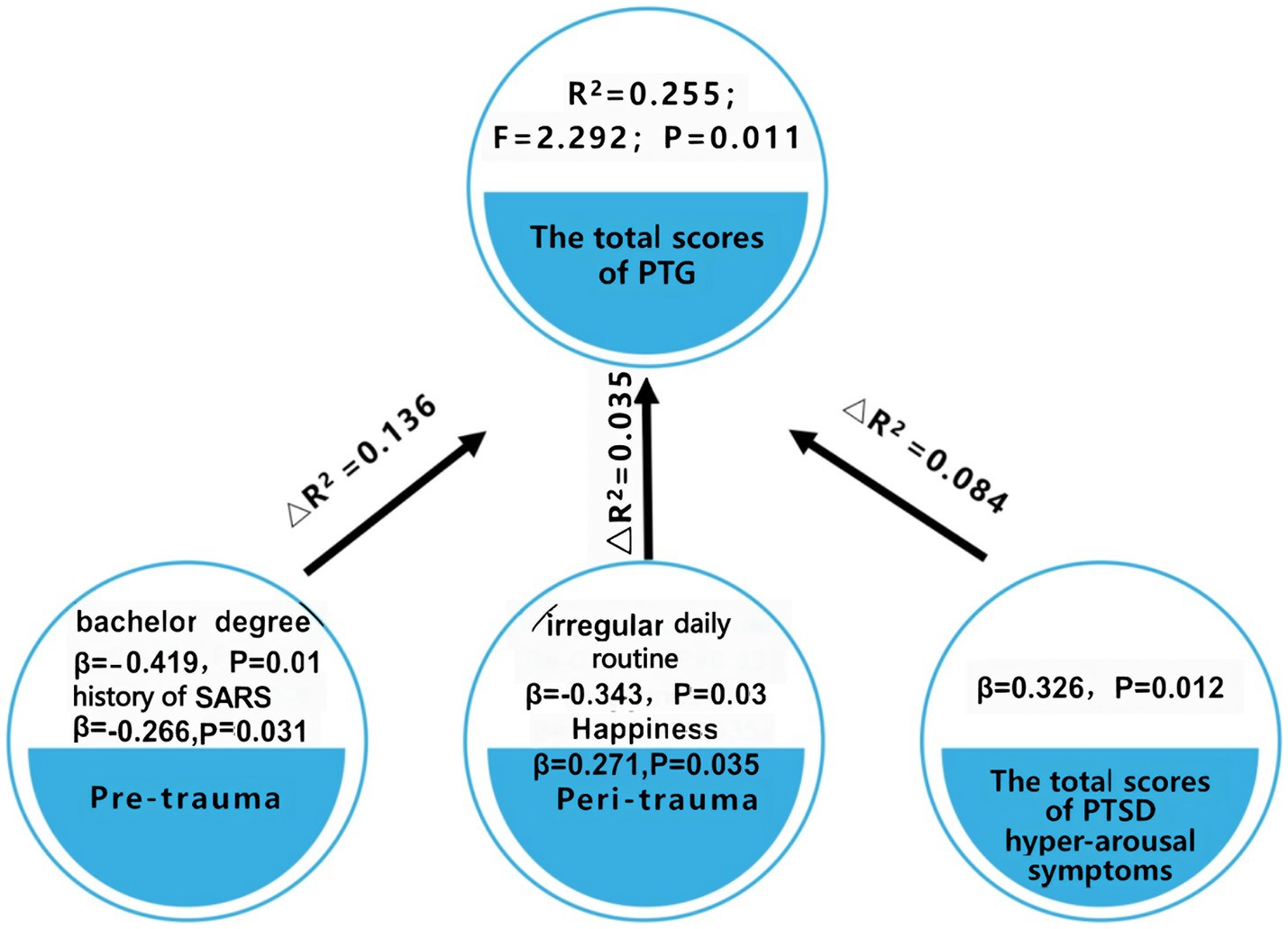
Figure 2 . Evaluation of PTG predictors in patients diagnosed with COVID-19 in Wuhan.
Notably, higher PTG scores were observed in patients exuding happiness (β = 0.271, p = 0.035) and those testing positive for hyperarousal symptoms of PTSD (β = 0.326, p = 0.012). In contrast, patients with a bachelor’s degree (compared to those with elementary schooling) (β = −0.419, p = 0.01), those with a history of SARS (β = −0.266, p = 0.031), and those with irregular daily routine (β = −0.343, p = 0.03) registered diminished PTG scores.
It is pertinent to underscore that the variables incorporated within this regression model elucidated only a fraction of the overall outcome. This suggests the probable influence of additional factors, which remain unexplored in this study, on the PTG scores, as depicted in Table 5 and Figure 2 .
4 Discussion
This study is one of the first to report the prevalence of hyper-arousal symptoms and PTG in inpatients with COVID-19 at Wuhan during their early days of hospitalization. These inpatients experienced strong psychological turbulence during the early hospitalization stage; i.e., more stress and more growth. The primary physiological symptoms pinpointed in these patients were an inconsistent daily routine and sleep disturbances. Both these manifestations were more pronounced than in healthy individuals during the same timeframe. On the emotional spectrum, a significant majority, 69.6%, exuded serenity, while negative emotions like anxiety, anger, melancholy, and fear were reported at lower rates (anxiety, 18.6%; anger, 2%; melancholy, 10.8%; and fear, 8.8%). The study conducted by Wang, which assessed suicidal tendencies, sleep conditions, and psychological health of COVID-19 patients, suggested an anxiety rate of 31.91%, a figure that diverges from our findings ( Wang et al., 2020 ). The finding indicates that inpatients experience a certain level of psychosocial responses at the early stage of hospitalization when doctors are likely to focus on physical treatment.
In the face of the COVID-19 pandemic, a major stress-inducing event, psychological crises of varying intensities have been observed across individuals and communities. Post-infection, a considerable number of patients exhibited heightened alertness, characterized by diminished concentration, disrupted sleep patterns, irritability, increased sensitivity, and increased startle response. Our study found that 37.3% of the hospitalized patients in Wuhan displayed hyperarousal symptoms of PTSD, surpassing the 13.1% in healthy counterparts during the same period. Similarly, a previous study investigated the psychiatric symptoms in patients who recovered from COVID-19 and reported that 25.4% of patients reported clinical PTSD ( Poyraz et al., 2021 ). One probable explanation is that our study concentrated on the early hospitalization stage, which may be characterized by more psychological trauma due to unfamiliar isolation treatment. In addition, during the first pandemic peak of the COVID-19 outbreak in Wuhan, during which the novel coronavirus was of unknown origin, aggressively contagious, lacked effective diagnostic and therapeutic protocols, and had a high morbidity and mortality rate. As a result, patients may have had a more intense fear of the acute hazards posed by the unknown virus, which could also have affected their psychological well-being. The most pressing symptom identified was disrupted sleep, accentuating the importance for healthcare practitioners to focus on sleep regulations for mitigating PTSD levels.
Defined by Tedeschi and Calhoun as the positive psychological metamorphosis post trauma, PTG was observed in 77.5% of the COVID-19 patients in our study ( Tedeschi and Calhoun, 1996 ). This surpassed the scores in the healthy demographic for the same timeframe. A PTG rate of 51.1% was previously reported among earthquake survivors ( Jin et al., 2014 ). Additionally, Calhoun and Tedeschi ( Calhoun and Tedeschi, 2014 ) concluded in a review that the prevalence of PTG in individuals ranged from 30 to 90%, which covers our findings. This elevation in PTG post the traumatic experience of the pandemic can be attributed to the ability of the patients to introspect, gain a renewed perspective on life, bolster personal resilience, and consequently attain higher PTG levels. Further, the most notable score was observed in interpersonal relationships, in line with the research conducted by Dong ( Dong, 2016 ). One possible explanation is that during the patient’s hospitalization and isolation, the patient is able to receive specialized care and assistance from healthcare professionals, which increases trust and dependence on others, ushering in greater emotional expression and proactive help-seeking behavior.
The results suggested that patients with COVID-19 in Wuhan had a more significant psychological turbulence during the early stage than the healthy demographic. Indeed, higher hyper-arousal and growth in patients showed that the patients had a rough time during the early hospitalization stage. However, it should be noted that, in the early stage of the COVID-19 outbreak, much emphasis was placed on the biological, epidemiological, and clinical characteristics of COVID-19, whereas limited research has addressed the psychological consequences among COVID-19 patients. The findings motivated healthcare workers to pay attention to trauma and growth responses in patients simultaneously, i.e., reducing psychological trauma while promoting psychological growth in interventions.
Utilizing the three-phase trauma theory, we analyzed the influence of various trauma phases on PTSD and PTG in patients. The results revealed that pre-trauma factors were less predictive of PTSD than those during trauma, this is consistent with previous findings that pre-traumatic factors are the weakest predictors of PTSD compared to pri-and post-traumatic factors. As inferred by the meta-analysis on children and adolescents conducted by Trickey, pre-trauma factors might only be indicative of PTSD in specific subsets, and their predictability can vary across different cohorts ( Trickey et al., 2012 ). This assertion warrants further research with more extensive sample sizes. In terms of PTG, both pre-trauma and pri-trauma variables held predictive value, whereas educational background and history of SARS exposure, as two pre-traumatic variables, are important factors influencing PTG.
Previous studies demonstrated that the subjective emotional response of a patient during trauma serves as a pivotal predictor of both PTSD and PTG ( Carlier et al., 1997 ). Consistent with this paradigm, the results of our study reaffirm the significance of this relationship. In particular, the experience of serenity and happiness during trauma—both signifiers of affirmative emotional responses—stand as robust protective factors against hyper-arousal symptoms of PTSD and concurrently bolster PTG. A salient observation is that hospitalized patients who retain feelings of serenity and happiness manifest lower scores pertaining to hyper-arousal symptoms of PTSD and higher scores for PTG. However, few studies investigated subjective emotional reactions throughout the pri-traumatic period, and most of them focused on negative feelings such as worry and terror ( Chen et al., 2021 ). Our study comprehensively analyzed the prediction of emotional responses on patients’ psychological well-being from the perspective of both positive and negative emotion, which is the first report of its kind. Though our findings could not validate adverse emotional responses of patients during trauma as predictive factors for hyper-arousal symptoms of PTSD and PTG, extant studies postulate that resilience-promoting factors can potentially illuminate corresponding risk factors ( Iacoviello Brian and Charney, 2014 ). Hence, we hypothesize that the adverse emotional response of patients during trauma might predict risks associated with hyper-arousal symptoms of PTSD and PTG, albeit this necessitates further empirical substantiation. Further, we found that an irregular sleep pattern during hospitalization emerged as a risk factor for hyper-arousal symptoms of PTSD and PTG. Those with inconsistent sleep manifested elevated hyper-arousal symptoms of PTSD and diminished PTG scores. Sun ( Sun et al., 2021 ) revealed a significant correlation between sleep quality and PTSD symptom levels in COVID-19 patients in his study, which is consistent with our results. Sleep disturbances were common among people involved in the COVID-19 pandemic ( Liu et al., 2020 ; Sun et al., 2021 ). Compared with PTSD, sleep disturbances are more likely to be recognized by clinicians. Timely identification and treatment for trauma-induced sleep problems may prevent the development of PTSD.
A noteworthy dimension of our findings was the realization that, concerning both hyper-arousal symptoms of PTSD and PTG, the identified variables in the regression analysis illuminated only a fragment of the model outcomes. This insinuates the likelihood of unaccounted factors influencing the psychological well-being of hospitalized patients diagnosed with COVID-19 in Wuhan. The focal point of our inquiry was the hospitalization period, primarily assessing pre-trauma and during-trauma factors. Prior studies emphasize the predictive potential of post-traumatic psychosocial factors, such as supportive social networks, optimism, cognitive adaptability, and affirmative beliefs, which potentially align with our findings and place emphasis on post-traumatic factors ( Sayed et al., 2015 ).
With PTSD and PTG epitomizing the dualistic outcomes post trauma—reflecting both detrimental impacts and positive growth trajectories—our findings infer that hyper-arousal symptoms of PTSD may be essential for PTG. Evidently, patients manifesting hyper-arousal symptoms of PTSD tend to register elevated PTG scores. This mirrors findings from previous studies of COVID-19 pandemic populations ( Bonazza et al., 2022 ; Collazo-Castiñeira et al., 2022 ). According to previous reports, post-traumatic development (spiritual transformation, appreciation of life, new possibilities, and personal strength) was positively associated with post-traumatic stress. The conjecture is that post traumatic occurrences, individuals may encounter heightened alertness reactions, inducing self-rumination and introspection, catalyzing core belief reformation, culminating in PTG ( Liu et al., 2017 ). Thus, while trauma may destabilize and challenge core beliefs, the ensuing dissonance, although disconcerting, could be a catalyst for growth.
5 Implications
This cross-sectional study examines the psychological distress experienced by early hospitalized patients during the first peak of the COVID-19 outbreak in Wuhan. The study is valuable for understanding the impact of the pandemic on patients’ mental health. The study found that certain variables during the trauma period, specifically irregular daily routines and positive perception of emotions, were strong predictors of hyper-arousal symptoms and post-traumatic growth (PTG). These variables were even stronger predictors than demographic variables, which are typically stable and immutable. This suggests that patients’ irregular daily routines and subjective perception of emotions during hospitalization have a significant impact on their psychological well-being. Is it possible to intervene early in a patient’s hospitalization to prevent PTSD after discharge and promote post-traumatic growth? Firstly, to improve patients’ positive emotions, health workers can help them adapt in three ways: (1) health workers should help patients realize that it is normal to experience negative emotions when going through a serious traumatic event. (2) Patients should be encouraged to adopt positive coping styles, such as communicating with family or friends or engaging in activities that interest and give pleasure to them. (3) Optional and optimal digital mental health services should be provided. For instance, an online counseling platform can facilitate hospitalized patients to seek help independently and relieve psychological stress. Secondly, to adjust the irregular daily routine of patients, the following measures can be taken: (1) Improving the ward environment by reducing noise levels, such as talking and alarms, and softening the lighting. (2) Providing patients with noise-canceling earplugs and light-blocking eye masks when they need to rest. (3) Hospitalized patients can benefit from a range of behavioral interventions, such as music therapy ( Majid et al., 2023 ), mindfulness meditation ( Johnson Leslie et al., 2023 ), and the most evidence-based treatment might be cognitive behavior therapy (CBT) ( Redeker Nancy, 2023 ), especially the Internet CBT that can prevent the spread of infection during the pandemic.
6 Limitations of this study
This study, while providing valuable insights, is not without its limitations. First, a primary constraint pertains to the exclusive focus on pre-trauma and pri-trauma factors in assessing the psychological health of patients. However, the results of our study allude to the potential significance of post-traumatic factors in influencing the psychological health of patients, thus carving a pathway for prospective research endeavors. Second, our sampling methodology excluded patients who faced challenges in utilizing communication tools or needed external assistance to navigate the online questionnaire. This potentially marginalizes a segment of the population, notably those with limited educational backgrounds. Given that education level surfaces as a potentially influential factors of psychological health, this exclusion could skew our findings. Future investigations would benefit from adopting a more inclusive approach, ensuring a holistic representation of the target demographic. Third, potential bias might exist in this study, such as selection bias in the recruitment of participants (the extremely severe patients were excluded whose psychological responses might be worse) and information bias in the participants’ psychological condition prior to admission (participants’ baseline psychological condition might influence their psychological responses during hospitalization). To reduce or avoid potential bias, clinical nurses and psychiatrists worked together to communicate with participants and collect information during the period of study design and conduction. Furthermore, data quality control was practiced rigidly during the whole process of study.
7 Conclusion
This research stands out due to its pioneering approach in coalescing PTSD and PTG, presenting them as intertwined facets of the post-traumatic psychological spectrum, and methodically delineating the psychological landscape of patients diagnosed with COVID-19 during their hospitalization in Wuhan. Drawing upon the pre-trauma, pri-trauma, and post-trauma conceptual framework, this study offers a comparative analysis of the predictive powers of factors across these trauma phases on the psychological health of patients. Of particular note is our exploration into the influence of subjective emotional experiences during trauma, framing it as a predictor for both PTSD and PTG from a constructively oriented viewpoint. This augments our comprehension of the intricate psychological responses that patients manifest in the wake of traumatic episodes. Such insights not only enrich the academic discourse around trauma-induced psychological responses but also establish a robust empirical foundation for crafting nuanced, enduring, and systematic therapeutic interventions tailored for the psychological trauma endured by patients diagnosed with COVID-19.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Army Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
J-jC: Conceptualization, Resources, Writing – original draft, Writing – review & editing. BY: Data curation, Formal analysis, Writing – original draft. LY: Data curation, Formal analysis, Writing – original draft. X-xS: Data curation, Formal analysis, Writing – review & editing. QD: Conceptualization, Methodology, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Research Center for Nursing Humanity (Fujian Medical University), the key project of natural science foundation of Chongqing (cstc2020jcyj-zdxmX0009), and the key project and innovation project of People’s Liberation Army of China (2021HL003).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. ^ https://www.wjx.cn
Arpawong, T. E., Oland, A., Milam, J. E., Ruccione, K., and Meeske, K. A. (2013). Post-traumatic growth among an ethnically diverse sample of adolescent and young adult cancer survivors. Psychooncology 22, 2235–2244. doi: 10.1002/pon.3286
PubMed Abstract | Crossref Full Text | Google Scholar
Barnett, J. H., Salmond, C. H., Jones, P. B., and Sahakian, B. J. (2006). Cognitive reserve in neuropsychiatry. Psychol. Med. 36, 1053–1064. doi: 10.1017/S0033291706007501
Crossref Full Text | Google Scholar
Bonazza, F., Luridiana Battistini, C., Fior, G., Bergamelli, E., Wiedenmann, F., D’Agostino, A., et al. (2022). Recovering from COVID-19: psychological sequelae and post-traumatic growth six months after discharge. Eur. J. Psychotraumatol. 13:2095133. doi: 10.1080/20008198.2022.2095133
Breslau, N., Reboussin, B. A., Anthony, J. C., and Storr, C. L. (2005). The structure of posttraumatic stress disorder: latent class analysis in 2 community samples. Arch. Gen. Psychiatry 62, 1343–1351. doi: 10.1001/archpsyc.62.12.1343
Calhoun, L. G., and Tedeschi, R. G. (2014). Handbook of posttraumatic growth: Research and practice . New York: Routledge. doi: 10.4324/9781315805597
Carlier, I. V., Lamberts, R. D., and Gersons, B. P. (1997). Risk factors for posttraumatic stress symptomatology in police officers: a prospective analysis. J. Nerv. Ment. Dis. 185, 498–506. doi: 10.1097/00005053-199708000-00004
Chen, R., Sun, C., Chen, J. J., Jen, H. J., Kang, X. L., Kao, C. C., et al. (2021). A large-scale survey on trauma, burnout, and posttraumatic growth among nurses during the COVID-19 pandemic. Int. J. Ment. Health Nurs. 30, 102–116. doi: 10.1111/inm.12796
Conybeare, D., Behar, E., Solomon, A., Newman, M. G., and Borkovec, T. D. (2012). The PTSD checklist-civilian version: reliability, validity, and factor structure in a nonclinical sample. J. Clin. Psychol. 68, 699–713. doi: 10.1002/jclp.21845
Collazo-Castiñeira, P., Rodríguez-Rey, R., Garrido-Hernansaiz, H., and Collado, S.. (2022). Prediction of post-traumatic growth in the face of the COVID-19 crisis based on resilience, post-traumatic stress and social participation: a longitudinal study. Front. Psychol. 13:985879. doi: 10.3389/fpsyg.2022.985879
Dekel, S., Mandl, C., and Solomon, Z. (2011). Shared and unique predictors of post-traumatic growth and distress. J. Clin. Psychol. 67, 241–252. doi: 10.1002/jclp.20747
Diagnosis and treatment (2020). Diagnosis and treatment plan of novel coronavirus pneumonia (trial 5th revised edition). Chin. J. Integr. Tradition. West. Med. 40, 136–138,
Google Scholar
Dong, L. H. (2016). Investigation on influencing factors of post-traumatic growth in peritoneal dialysis patients and intervention of group rational emotive therapy . Zhejiang: Zhejiang Chinese Medicine University.
Hong, X., Currier, G. W., Zhao, X., Jiang, Y., Zhou, W., and Wei, J. (2009). Posttraumatic stress disorder in convalescent severe acute respiratory syndrome patients: a 4-year follow-up study. Gen. Hosp. Psychiatry 31, 546–554. doi: 10.1016/j.genhosppsych.2009.06.008
Iacoviello Brian, M., and Charney, D. S. (2014). Psychosocial facets of resilience: implications for preventing posttrauma psychopathology, treating trauma survivors, and enhancing community resilience. Eur. J. Psychotraumatol. 5:undefined. doi: 10.3402/ejpt.v5.23970
Jin, Y., Xu, J., and Liu, D. (2014). The relationship between post traumatic stress disorder and post traumatic growth: gender differences in PTG and PTSD subgroups. Soc. Psychiatry Psychiatr. Epidemiol. 49, 1903–1910. doi: 10.1007/s00127-014-0865-5
Jin, Y., Xu, J., Liu, H., and Liu, D. (2014). Posttraumatic stress disorder and posttraumatic growth among adult survivors of Wenchuan earthquake after 1 year: prevalence and correlates. Arch. Psychiatr. Nurs. 28, 67–73. doi: 10.1016/j.apnu.2013.10.010
Johnson, L. C. M., Aiello, J. J., Jagtiani, A., Moore, K. N., Barber, L., Gujral, U. P., et al. (2023). Feasibility, appropriateness, and acceptability of a mobile mindfulness meditation intervention to improve sleep quality among a racially/ethnically diverse population. Sleep Health 9, 196–202. doi: 10.1016/j.sleh.2022.09.014
Kessler, R. C., Berglund, P., Demler, O., Jin, R., Merikangas, K. R., and Walters, E. E. (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 593–602. doi: 10.1001/archpsyc.62.6.593
Klosky, J. L., Krull, K. R., Kawashima, T., Leisenring, W., Randolph, M. E., Zebrack, B., et al. (2014). Relations between posttraumatic stress and posttraumatic growth in long-term survivors of childhood cancer: a report from the childhood Cancer survivor study. Health Psychol. 33, 878–882. doi: 10.1037/hea0000076
Lee, S. M., Kang, W. S., Cho, A. R., Kim, T., and Park, J. K. (2018). Psychological impact of the 2015 MERS outbreak on hospital workers and quarantined hemodialysis patients. Compr. Psychiatry 87, 123–127. doi: 10.1016/j.comppsych.2018.10.003
Li, Q., Guan, X., Wu, P., Wang, X., Zhou, L., Tong, Y., et al. (2020). Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 382, 1199–1207. doi: 10.1056/NEJMoa2001316
Liu, A. N., Wang, L. L., Li, H. P., Gong, J., and Liu, X. H. (2017). Correlation between posttraumatic growth and posttraumatic stress disorder symptoms based on Pearson correlation coefficient: a Meta-analysis. J. Nerv. Ment. Dis. 205, 380–389. doi: 10.1097/NMD.0000000000000605
Liu, N., Zhang, F., Wei, C., Jia, Y., Shang, Z., Sun, L., et al. (2020). Prevalence and predictors of PTSS during the COVID-19 outbreak in China hardest-hit areas: gender differences matter. Psychiatry Res. 287:112921. doi: 10.1016/j.psychres.2020.112921
Majid, D., Elham, A., and Mona, H. (2023). The effect of music on the improvement of sleep quality: a report from the viewpoint of Jorjani. Neurol. Sci. 44, 1787–1789. doi: 10.1007/s10072-023-06702-2
McGrath, J. J., Saha, S., Al-Hamzawi, A., Alonso, J., Bromet, E. J., Bruffaerts, R., et al. (2015). Psychotic experiences in the general population: a cross-National Analysis Based on 31, 261 respondents from 18 countries. JAMA Psychiatry 72, 697–705. doi: 10.1001/jamapsychiatry.2015.0575
Ozer, E. J., Best, S. R., Lipsey, T. L., and Weiss, D. S. (2003). Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychol. Bull. 129, 52–73. doi: 10.1037/0033-2909.129.1.52
Poyraz, B. A., Poyraz, C. A., Olgun, Y., and Gürel, Z. (2021). Psychiatric morbidity and protracted symptoms after COVID-19. Psychiatry Res. 295:113604. doi: 10.1016/j.psychres.2020.113604
Redeker Nancy, S. (2023). O'Connell Meghan, Conley Samantha, response to letter to the editor on: interpreting the effectiveness of cognitive behavioural therapy (CBT) and self-management strategies in heart failure patients for improving sleep', in response to the article-'coping, symptoms, and insomnia among people with heart failure during the COVID-19 pandemic. Eur. J. Cardiovasc. Nurs. 22, e27–e28. doi: 10.1093/eurjcn/zvad016
Sareen, J., Cox, B. J., Afifi TOde Graaf, R., Asmundson, G. J., ten Have, M., et al. (2005). Anxiety disorders and risk for suicidal ideation and suicide attempts: a population-based longitudinal study of adults. Arch. Gen. Psychiatry 62, 1249–1257. doi: 10.1001/archpsyc.62.11.1249
Sayed, S., Iacoviello, B. M., and Charney, D. S. (2015). Risk factors for the development of psychopathology following trauma. Curr. Psychiatry Rep. 17:612. doi: 10.1007/s11920-015-0612-y
Sun, L., Sun, Z., Wu, L., Zhu, Z., Zhang, F., Shang, Z., et al. (2021). Prevalence and risk factors for acute posttraumatic stress disorder during the COVID-19 outbreak. J. Affect. Disord. 283, 123–129. doi: 10.1016/j.jad.2021.01.050
Sun, L., Yi, B., Pan, X., Wu, L., Shang, Z., Jia, Y., et al. (2021). PTSD symptoms and sleep quality of COVID-19 patients during hospitalization: an observational study from two centers. Nat. Sci. Sleep 13, 1519–1531. doi: 10.2147/NSS.S317618
Tedeschi, R. G., and Calhoun, L. G. (1996). The posttraumatic growth inventory: measuring the positive legacy of trauma. J. Trauma. Stress. 9, 455–471. doi: 10.1002/jts.2490090305
Tedeschi, R. G., and Calhoun, L. G. (2004). TARGET ARTICLE: "posttraumatic growth: conceptual foundations and empirical evidence". Psychol. Inq. 15, 1–18. doi: 10.1207/s15327965pli1501_01
Tomita, M., Takahashi, M., Tagaya, N., Kakuta, M., Kai, I., and Muto, T. (2017). Structural equation modeling of the relationship between posttraumatic growth and psychosocial factors in women with breast cancer. Psychooncology 26, 1198–1204. doi: 10.1002/pon.4298
Trickey, D., Siddaway, A. P., Meiser-Stedman, R., Serpell, L., and Field, A. P. (2012). A meta-analysis of risk factors for post-traumatic stress disorder in children and adolescents. Clin. Psychol. Rev. 32, 122–138. doi: 10.1016/j.cpr.2011.12.001
Wang, C., Horby, P. W., Hayden, F. G., and Gao, G. F. (2020). A novel coronavirus outbreak of global health concern. Lancet 395, 470–473. doi: 10.1016/S0140-6736(20)30185-9
Wang, R. J., Li, J., Mei, J. H., Xiao, M. Z., Kuang, L., Song, B., et al. (2020). Suicide risk, sleep, psychological status and influencing factors in patients with novel coronavirus pneumonia. J. Third Milit. Med. Univ. 42, 1462–1468. doi: 10.16016/j.1000-5404.202003229
Keywords: COVID-19, physical and psychological conditions, post-traumatic growth (PTG), post-traumatic stress disorder (PTSD), nursing
Citation: Chen J-j, Yu B, Yan L, Sun X-x and Dai Q (2024) A study on post-traumatic stress disorder and post-traumatic growth among patients infected with COVID-19 in Wuhan. Front. Psychol . 15:1343264. doi: 10.3389/fpsyg.2024.1343264
Received: 05 December 2023; Accepted: 16 April 2024; Published: 16 May 2024.
Reviewed by:
Copyright © 2024 Chen, Yu, Yan, Sun and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qin Dai, [email protected]
† These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.351; 2015
Post-traumatic stress disorder
What you need to know.
- Individual reactions to traumatic events vary greatly and most people do not develop a mental disorder after exposure to trauma
- PTSD should be considered in any patient exposed to a major traumatic event
- Up to 3% of adults has PTSD at any one time. Lifetime prevalence rates are between 1.9% and 8.8%
- Psychological treatments, particularly trauma focused psychological therapies, can be effective
- Although the effect sizes are not as high as for psychological therapies, drug treatments can be effective
- Patients with complex PTSD should receive specialist multidisciplinary care
What is post-traumatic stress disorder (PTSD)?
PTSD is a mental disorder that may develop after exposure to exceptionally threatening or horrifying events. Many people show remarkable resilience and capacity to recover following exposure to trauma. 1 PTSD can occur after a single traumatic event or from prolonged exposure to trauma, such as sexual abuse in childhood. Predicting who will go on to develop PTSD is a challenge. 2

Examples of the many different trajectories of PTSD symptoms after exposure to trauma
Sources and selection criteria
We identified Cochrane and other relevant systematic reviews and meta-analyses, and supplemented these with additional searches and our knowledge of the subject. Wherever possible, we used evidence from recent meta-analyses of randomised trials.
Patients with PTSD are at increased risk of experiencing poor physical health, including somatoform, cardiorespiratory, musculoskeletal, gastrointestinal, and immunological disorders. 3 4 It is also associated with substantial psychiatric comorbidity, 5 increased risk of suicide, 6 and considerable economic burden. 7 8
PTSD is a widely accepted diagnosis 9 but some believe that the term medicalises understandable responses to catastrophic events and further disempowers those who are already disempowered. 10
How common is PTSD?
About 3% of the adult population has PTSD at any one time. 11 Lifetime prevalence is between 1.9% 12 and 8.8%, 7 but this rate doubles in populations affected by conflict 13 and reaches more than 50% in survivors of rape. 5
How does PTSD present?
Symptoms include persistent intrusive recollections, avoidance of stimuli related to the trauma, negative alterations in cognitions and mood, and hyperarousal (table (table ). 14 15 A diagnosis can be made in someone whose ability to function normally has been noticeably impaired for one month according to DSM-5 criteria. Delayed presentation (sometimes years later) is common, 7 including where the effects are severe. 16
Symptoms required for diagnosis of PTSD
How is PTSD diagnosed?
Box 1 describes the nature of the traumatic event(s) required by DSM-5 (diagnostic and statistical manual of mental disorders, fifth edition) 14 for diagnosis and the proposed criteria by ICD-11 (international classification of diseases, 11th revision). 17 Some events such as bullying, divorce, death of a pet, and learning about a diagnosis of cancer in a close family member are not deemed extreme enough to precipitate PTSD. However, they can result in almost identical symptoms and raise questions about the validity of the definitions for traumatic events. 18
Box 1 Traumatic event(s) required for diagnosis of PTSD
Dsm-5 criteria[14].
- Directly experiencing the traumatic event(s)
- Witnessing traumatic event(s) in others
- Learning that the traumatic event(s) occurred to a close family member or close friend; cases of actual or threatened death must have been violent or unintentional
- Experiencing repeated or extreme exposure to aversive details of the traumatic event(s) (for example, first responders collecting human remains; police officers repeatedly exposed to details of child abuse); this does not apply to exposure through electronic media, television, movies, or pictures, unless this exposure is work related
Proposed ICD-11 criterion[17]
- Exposure to an extremely threatening or horrific event or series of events
DSM-5 lists the 20 symptoms required for PTSD to be diagnosed, 14 separated into four groups (table). All symptoms must be associated with the traumatic event. In the proposed criteria by ICD-11, 17 PTSD will be diagnosed according to six criteria (table). To reflect the heterogeneity of PTSD, ICD-11 will introduce a new complex PTSD diagnosis (table). This requires satisfaction of the criteria for PTSD plus symptoms of mood dysregulation, negative self concept, and persistent difficulty in sustaining relationships and feeling close to others. Service users may meet the diagnostic criteria in one system but not in the other owing to the differences. 19
Can PTSD be prevented?
Psychological interventions.
Psychological interventions have been evaluated after traumas concerning a single incident, such as a road traffic crash and physical or sexual assaults. Meta-analyses show that brief, trauma focused, cognitive behavioural interventions can reduce the severity of symptoms when the intervention is targeted at those with early symptoms. 20 21 However, non-targeted interventions (including psychoeducation, psychological debriefing, individual and group counselling, cognitive behavioural therapy (CBT) based programmes, and collaborative care based approaches) are largely ineffective. 22 23 24 25
Drug interventions
No robust evidence supports the use of drug interventions. 26
Prevention after large scale traumatic events
Evidence to support routine intervention after traumatic events involving many people (for example, terrorist attacks and natural disasters) is lacking. However, some evidence suggests that high levels of social support are perceived as protective. 27 Consensus guidelines recommend supportive, practical, and pragmatic input but avoidance of formal clinical interventions unless indicated. 28 29 30
Can PTSD be treated?
Psychological therapy.
Clinical guidelines recommend trauma focused psychological therapies based on evidence from systematic reviews and meta-analyses. 31 32 33 Individual trauma focused CBT and eye movement desensitisation and reprocessing (EMDR) (box 2) have been found to be equally effective. 34
Box 2 Trauma focused exposure therapy, CBT and EMDR
Exposure therapy.
- Therapists help patients to confront their traumatic memories through written or verbal narrative, detailed recounting of the traumatic experience, and repeated exposure to trauma related situations that were being avoided or evoked fear but are now safe (for example, driving a car where the road traffic incident occurred or walking in the busy park where an assault occurred)
Cognitive therapy
- Focuses on identifying and modifying misinterpretations that led patients to overestimate the current threat (for example, patients who think assault is almost inevitable if they leave the house)
- Focuses on modifying beliefs and how patients interpret their behaviour during the trauma, including problems with guilt and shame
- Standardised, trauma focused procedure. Involves the use of bilateral physical stimulation (eye movements, taps, or tones), hypothesised to stimulate the patient’s information processing to help integrate the targeted event as an adaptive contextualised memory
Group trauma focused CBT is also effective, but fewer studies have focused on this method. 35 Non-trauma focused CBT—including components such as grounding techniques to manage flashbacks (for example, focusing on the here and now by describing items in a room), relaxation training (for example, controlled breathing and progressive muscle relaxation), positive thinking and self talk (for example, repeating positive phrases such as “I can deal with this”)—has been found to be superior to waiting list control groups and has shown similar efficacy to trauma focused CBT and EMDR immediately after treatment, but this is not maintained at follow-up. 34 Non-trauma focused CBT offers a valid alternative to trauma focused therapy if the latter is poorly tolerated, contraindicated, or unavailable. It is unclear whether specific therapies are more or less effective for particular subgroups or trauma types. 36 37
Research on interventions for more complex presentations of PTSD is limited. 38 Evidence suggests that phased approaches may be beneficial for more complex presentations of PTSD. 39 Phase based approaches target problems such as affect dysregulation, dissociation, and somatic symptoms to promote adaptive coping, a sense of safety, and stabilisation before undertaking any trauma focused intervention.
Self help programmes
Guided self help interventions for depression and anxiety disorders are being used as an alternative to face to face therapy as these interventions offer enhanced access to cost effective treatment. 40 Some evidence suggests that internet based guided self help therapies effectively alleviate the symptoms of traumatic stress, but randomised controlled trials (RCTs) have historically been limited to subsyndromal populations. 41 42 More recent evidence supports the efficacy of guided self help for people meeting diagnostic criteria for PTSD, 43 44 45 but no head to head trials have compared guided self help with trauma focused psychological therapy administered by a therapist.
Drug treatment
The National Institute for Health and Care Excellence and World Health Organization recommend drug treatment second to trauma focused therapy. 33 46 The effect sizes for drug treatments compared with placebo are inferior to those reported for psychological treatments with a trauma focus over waiting list or treatment as usual controls. 33 47 Effect sizes with drug treatment are similar to those observed from use of antidepressants for depression compared with placebo. 48 A recent systematic review and meta-analysis found statistically significant evidence (when at least two RCTs were available) of reduction in severity of PTSD symptoms for four drugs (fluoxetine, paroxetine, sertraline, and venlafaxine) versus placebo. 47 In single RCTs, amitriptyline, {"type":"entrez-nucleotide","attrs":{"text":"GR205171","term_id":"238470896","term_text":"GR205171"}} GR205171 (a neurokinin-1 antagonist), mirtazapine, and phenelzine have shown superiority over placebo in reducing the symptoms of PTSD.
In an RCT the α 1 adrenoceptor antagonist prazosin was found to reduce nightmares in veterans with PTSD, 49 and a further RCT in veterans showed reduction in overall symptom severity. 50 This suggests a possible role for α 1 adrenoceptor blockers in PTSD, although further research is needed. Olanzapine, in contrast with another antipsychotic, risperidone, has been shown to accentuate the effects of antidepressants when resistance to treatment is encountered. 51 52
Combination therapy
Evidence to support the use of pharmacotherapy combined with psychological therapy over either treatment method separately is insufficient. 53
How should PTSD and comorbidity be managed?
PTSD is associated with depression, anxiety disorders, and drug and alcohol use disorders. Little evidence exists for the effectiveness of psychological interventions for PTSD with comorbid substance use disorders. Some evidence suggests that trauma focused CBT can be effective with concomitant interventions to stabilise drug or alcohol use, but treatment effects are not as large as for PTSD in the absence of drug or alcohol misuse. 54
What is the prognosis in PTSD?
Few longitudinal follow-up studies have been done of PTSD, but for many patients PTSD is severe and enduring. 5 There is, however, good evidence that patients may benefit from treatment even when the symptoms have been present for many years. 34
Are there emerging options to prevent and treat PTSD?
Several experimental studies provide hope that better or alternative ways to prevent and treat PTSD are on the way. Simple visuospatial tasks such as playing a computer game shortly after a traumatic experience reduce re-experiencing. 55 For established PTSD, interest in using drugs to augment psychological therapy is increasing. The results of a recent RCT of the psychedelic 3,4-methylenedioxymethylamphetamine with psychotherapy for treatment resistant PTSD have been promising. 56 57 These approaches remain in their infancy, and further well designed clinical studies are required to determine if they will live up to their early promise.
How were patients involved in this clinical review?
Sarah Cosgrove is a former patient with PTSD and a representative of the public in Cardiff University’s Traumatic Stress Research Group. Sarah is a coauthor of the paper and provides an account of her experiences in the patient’s perspective box.
A patient’s perspective
I was diagnosed with PTSD in November 2013 in the aftermath of a violent assault. From the time of the attack to the case coming to court, I had support from police and victim services enabling me to face my assailant in court with courage and conviction.
But in the weeks after the judicial process had concluded, I started to unravel. Naturally a glass half full sort of person, I slid into a state of great anxiety, frightened to be alone, scared to be in a group, reluctant to go out, and terrified of staying at home. I knew something was very wrong. I had gone from being confident and outgoing, to not being able to sleep, being tearful, and experiencing episodes of unparalleled low mood. My GP immediately diagnosed PTSD. Being able to put a label on what I was going through was so helpful—it meant that there was something wrong.
Fortunately, I was offered the chance to participate in a trial of a guided self help programme for sufferers of PTSD. This enabled me to both confront my experience and desensitise it, and within a few months I felt stronger than I had ever been. The programme has given me a coping strategy to employ whenever I get negative thoughts or flashbacks. It may have saved my life; at the very least it got me back to the person I used to be.
Tips for non-specialists
- A traumatic event can precipitate conditions other than PTSD, such as depression, phobic anxiety, and substance use disorders
- PTSD is associated with comorbidity
- Sensitive questioning is required to elicit symptoms of PTSD as patients may avoid volunteering their traumatic experience(s)
- Patients with PTSD may present in primary care with physical symptoms that are difficult to explain
- Trauma focused psychological therapy is the treatment of choice for PTSD, although drugs and other forms of psychological treatment can help
- Patient choice and availability of psychological therapy will influence the treatment given
When to suspect PTSD
- When patients present with mental or physical symptoms that cannot be fully explained after a traumatic event
- When patients present with characteristic symptoms of PTSD—re-experiencing, avoidance, and hyperarousal
- When patients disclose a history of involvement in a traumatic event
- When patients present with mental or physical symptoms that are difficult to explain in the absence of a disclosed traumatic event
Additional educational resources
Information for healthcare professionals.
- International Society for Traumatic Stress Studies ( www.istss.org )
- US Department of Veterans Affairs, National Centre for PTSD ( www.ptsd.va.gov )
Information for patients
- NHS Choices PTSD ( www.nhs.uk/Conditions/Post-traumatic-stress-disorder/Pages/Introduction.aspx )
- Royal College of Psychiatrists ( www.rcpsych.ac.uk/expertadvice/problemsdisorders/posttraumaticstressdisorder.aspx )
- National Centre for Mental Health ( http://ncmh.info/conditions/post-traumatic-stress-disorder-ptsd/ )
- US Department of Veterans Affairs, National Centre for PTSD ( www.ptsd.va.gov/public/pages/fslist-self-help-cope.asp )—provides information on the symptoms of PTSD, self help, and treatment options
Contributors: JB, CL, and NR planned, conducted reviews, and drafted the article. SC drafted the patient’s perspective box and commented on and amended the initial draft. All authors reviewed and agreed the final draft. JB is the guarantor.
Competing interests: We have read and understood the BMJ policy on declaration of interests and declare the following: JB, CL, and NR have undertaken systematic reviews, meta-analyses, randomised controlled trials, and other research in the specialty of traumatic stress, some of which is referred to in the manuscript. JB, CL, and NR are members of a research team that developed a web based guided self help programme to treat PTSD. The programme is likely to be marketed in the future. Royalties will be payable to Cardiff University, with a proportion of these being shared with the research team in line with Cardiff University’s rules.
Provenance and peer review: Commissioned; externally peer reviewed.
Cite this as: BMJ 2015;351:h6161

IMAGES
VIDEO
COMMENTS
Post-traumatic stress disorder (PTSD) is a psychiatric disorder that results from the experience or witnessing of traumatic or life-threatening events. PTSD has profound psychobiological correlates, which can impair the person's daily life and be life threatening. In light of current events (e.g. extended combat, terrorism, exposure to certain ...
Although traumatic stress has been known for over 100 years by a number of terms, including "shell shock", "battle fatigue", or "soldier's heart" 1, it was only in the 1980s that persistent stress reactions were recognized in psychiatric nosology.In the wake of the mental health problems evident in many troops returning from deployment in Vietnam, the DSM-III introduced the ...
Posttraumatic stress disorder (PTSD) is a chronic impairment disorder that occurs after exposure to traumatic events. This disorder can result in a disturbance to individual and family functioning, causing significant medical, financial, and social problems. This study is a selective review of literature aiming to provide a general outlook of the current understanding of PTSD.
Current treatment strategies for control of trauma-associated symptoms of Post Traumatic Stress Disorder (PTSD) have recently been updated by the Veterans Affairs (VA) and the Department of Defense (DoD, after over a decade of dedicated research. The most recent evidence is compelling that its use of trauma-focused therapies such as Cognitive ...
Post-traumatic stress disorder (PTSD) is one of the few neuropsychiatric disorders for which the timing and cause of onset are understood, facilitating research into the underlying mechanisms. In ...
PTSD is a mental disorder that may develop after exposure to. exceptionally threatening or horrifying events. Many people. show remarkable resilience and capacity to recover following. exposure to ...
Post-traumatic stress disorder is a common condition that affects millions of people across the world. ... This paper concludes that the quality of the evidence remains low but includes recommendations for cognitive behavioural therapy with a trauma focus (CBT-TF), cognitive therapy and eye movement desensitisation and reprocessing (EMDR) for ...
Introduction. Post-traumatic stress disorder (PTSD) is a possible psychopathological consequence of exposure to a traumatic event that threatens one's psychological and/or physical integrity Citation 1-3.Individuals with PTSD may recurrently experience memories, sensations, and emotions of a traumatic event through sensory flashbacks or nightmares, or experience avoidance, irritability ...
About This Journal. Journal of Traumatic Stress is an interdisciplinary forum for the publication of peer-reviewed original papers on biopsychosocial aspects of trauma. Papers focus on theoretical formulations, research, treatment, prevention education/training, and legal and policy concerns. It is the official publication of the International ...
Abstract. This narrative state-of-the-art review paper describes the progress in the understanding and treatment of Posttraumatic Stress Disorder (PTSD). Over the last four decades, the scientific landscape has matured, with many interdisciplinary contributions to understanding its diagnosis, etiology, and epidemiology.
Post-traumatic stress disorder (PTSD) can become a chronic and severely disabling condition resulting in a reduced quality of life and increased economic burden. The disorder is directly related to exposure to a traumatic event, e.g., a real or threatened injury, death, or sexual assault. Extensive research has been done on the neurobiological alterations underlying the disorder and its ...
Post-traumatic stress disorder (PTSD) is a serious neuropsychiatric condition affecting approximately 5% of the US population each year 1.Managing PTSD is particularly complicated in individuals ...
In this In Review paper, I discuss some of the important advances in PTSD. ... there is a need for longitudinal research on the course and predictors of ... et al. Selective serotonin reuptake inhibitors in the treatment of post-traumatic stress disorder: a meta-analysis of randomized controlled trials. Int Clin Psychopharmacol. 2000; 15 (Suppl ...
Post-traumatic stress disorder (PTSD) is a chronic and debilitating disorder characterized by hypervigilance and recurrent, intrusive memories of the traumatic event. Estimates for lifetime prevalence of PTSD diagnoses for United States military personnel vary widely from 10% to more than 30%.
Since post-traumatic stress disorder (PTSD) was introduced as a DSM diagnosis in 1980, what counts as traumatic events and symptoms has been continually contested and modified. Childhood maltreatment is now considered a form of interpersonal trauma involving physical, sexual, or emotional abuse, or neglect, but understandings are still shifting.
Posttraumatic stress disorder (PTSD) is a chronic psychological disorder that can develop after exposure to a traumatic event. This review summarizes the literature on the epidemiology, assessment, and treatment of PTSD. We provide a review of the characteristics of PTSD along with associated risk factors, and describe brief, evidence-based ...
Overview of psychological trauma, post-traumatic stress disorder, and biological markers. Psychological trauma can result from witnessing an event that is perceived to be life-threatening or to pose the potential of serious bodily injury to self or others. Such experiences, which are often accompanied by intense fear, horror, and helplessness ...
1. Introduction. Post-traumatic stress disorder (PTSD) is a severe psychiatric condition affecting approximately 3.5% of adults every year and has a lifetime prevalence of about 7% [Citation 1].PTSD is characterized by a heterogeneous symptom complex that involves intrusions (nightmares, dissociation, intrusive thoughts), avoidant behaviors, cognitive distortions, alterations of memory and ...
Post‐traumatic stress disorder (PTSD) is arguably the most common psychiatric disorder to arise after exposure to a traumatic event. Since its formal introduction in the DSM‐III in 1980, knowledge has grown significantly regarding its causes, maintaining mechanisms and treatments. Despite this increased understanding, however, the actual ...
Some people develop post-traumatic stress disorder (PTSD) after experiencing a shocking, scary, or dangerous event. It is natural to feel afraid during and after a traumatic situation. Fear is a part of the body's normal "fight-or-flight" response, which helps us avoid or respond to potential danger. People may experience a range of ...
Background: Post-traumatic stress disorder (PTSD) is the clinical manifestation of traumatic events and is associated with sleep disturbances. Sleep disturbances, if left untreated, may perpetuate or even worsen symptoms of PTSD. Previous studies of other PTSD populations show a higher incidence of sleep impairments and sleep disorders compared to healthy controls (HCs); however, this has ...
Research with patients who develop PTSD in the context of mild traumatic brain injury further suggests that PTSD may account for some or all of a patient's subjective cognitive complaints and neuropsychological test performance. Recommendations are provided for assessing PTSD in the context of COVID-19.
A study on post-traumatic stress disorder and post-traumatic growth among patients infected with COVID-19 in Wuhan Jing-jing Chen 1,2 † Bing Yu 3 † Ling Yan 4 Xiao-xiao Sun 2 Qin Dai 2 * 1 School of Nursing, Fujian Medical University, Research Center for Nursing Humanity, Fuzhou, Fujian, China
Sarah Cosgrove is a former patient with PTSD and a representative of the public in Cardiff University's Traumatic Stress Research Group. Sarah is a coauthor of the paper and provides an account of her experiences in the patient's perspective box. ... Post-traumatic stress disorder (PTSD): the management of PTSD in adults and children in ...
This study identified that SKA2 did not show association with risk of Major Depression Disorder and predicted that I22S, I22G, I78T, A15L, D18R, R25L, N42I, Y21S, K14I, K14L, and L60R were the most structurally and functionally significant nsSNPs in SKA2. Genes involved in the hypothalamic-pituitary-adrenal axis may be a robust biomarker of psychiatric disorders. Genetic polymorphisms of the ...
Art therapy has a long history in the work with trauma-related difficulties including post-traumatic stress disorder. The current literature review is the largest of its kind summarising 20 research papers on the impact of visual art therapy with adult trauma survivors. Themes identified across papers pertained to the impact on symptoms ...